Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous TiO2 Nanofibers (PTFs)
2.2. Preparation of Dense TiO2 Nanofibers (DTFs)
2.3. Preparation of Anatage TiO2 Nanoparticles (TNPs)
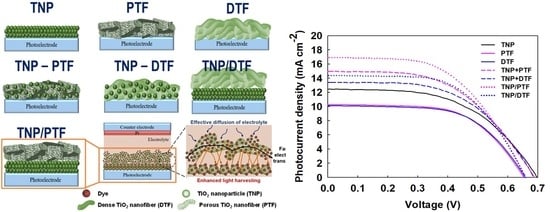
2.4. Preparation of DSSCs
2.5. Characteristic and Measurements
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tehare, K.K.; Navale, S.T.; Stadler, F.J.; He, Z.; Yang, H.; Xiong, X.; Liu, X.; Mane, R.S. Enhanced DSSCs Performance of TiO2 Nanostructure by Surface Passivation Layers. Mater. Res. Bull. 2018, 99, 491–495. [Google Scholar] [CrossRef]
- Giovannetti, R.; Zannotti, M.; Alibabaei, L.; Ferraro, S. Equilibrium and Kinetic Aspects in the Sensitization of Monolayer Transparent TiO2 Thin Films with Porphyrin Dyes for DSSC Applications. Int. J. Photoenergy 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Giannouli, M.; Govatsi, K.; Syrrokostas, G.; Yannopoulos, S.N.; Leftheriotis, G. Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. Nanoparticles. Materials 2018, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Naveen Kumar, S.K. Preparation and Thickness Optimization of TiO2/Nb2O5 Photoanode for Dye Sensitized Solar Cells. Mater. Today 2018, 5, 10797–10804. [Google Scholar] [CrossRef]
- Sasikumar, R.; Chen, T.-W.; Chen, S.M.; Rwei, S.-P.; Ramaraj, S.K. Developing the Photovoltaic Performance of Dye-sensitized Solar Cells (DSSCs) Using a SnO2-Doped Graphene Oxide Hybrid Nanocomposite as a Photo-Anode. Opt. Mater. 2018, 79, 345–352. [Google Scholar] [CrossRef]
- Jin, E.M.; Jeong, S.M.; Kang, H.-C.; Gu, H.-B. Photovoltaic Effect of Metal-Doped TiO2 Nanoparticles for Dye-Sensitized Solar Cells. ECS J. Solid State Sci. Technol. 2016, 5, Q109–Q114. [Google Scholar] [CrossRef]
- Kim, D.-W.; Jeong, Y.-B.; Kim, S.-H.; Lee, D.-Y.; Song, J.-S. Photovoltaic Performance of Dye-Sensitized Solar Cell Assembled with Gel Polymer Electrolyte. J. Power Sources 2005, 149, 112–116. [Google Scholar] [CrossRef]
- Palomares, E.; Clifford, J.N.; Haque, S.A.; Lutz, T.; Durrant, J.R. Slow Charge Recombination in Dye-Sensitized Solar Cells (DSSC) Using Al2O3 Coated Nanoporous TiO2 Films. Chem. Commun. 2002, 14, 1464–1465. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, A.; Jana, A.; Datta, J. Synthesis and Characterization of CdS Canoparticles Decorated TiO2 Matrix for an Efficient N3 Based Dye Sensitized Solar Cell (DSSC). J. Mater. Sco. Mater. Electron. 2016, 27, 12438–12445. [Google Scholar] [CrossRef]
- Yue, G.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M.; Fan, L.; Yao, Y. A Dye-Sensitized Solar Cell Based on PEDOT: PSS Counter Electrode. Chin. Sci. Bull. 2013, 58, 559–566. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-Efficient Dye-Sensitized Solar Cells With Collaborative Sensitization by Silyl-Anchor and Carboxyl-Anchor Dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kang, M. The Optical Properties of Nanoporous Structured Titanium Dioxide and the Photovoltaic Efficiency on DSSC. Mater. Chem. Phys. 2010, 122, 284–289. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Li, J.; Pan, M.; Bai, L.; Luoshan, M.; Zhao, X. Graphene Quantum Dots Optimization of Dye-Sensitized Solar Cells. Electrochim. Acta 2014, 137, 634–638. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on Dye-Sensitized Solar Cells (DSSCs): Advanced Techniques and Research Trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Wang, L.; Jia, L.; Li, Q. A Novel Sulfur Source for Biosynthesis of (Ag, S)-Modified TiO2 Photoanodes in DSSC. Mater. Lett. 2014, 123, 83–86. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, Y.; Kim, Y.; Kang, M. Enhanced Photoelectric Efficiency by Surface Modification of TiO2 Thin Film Using Various Acidic Species. Korean J. Chem. Eng. 2010, 27, 1462–1468. [Google Scholar] [CrossRef]
- Jin, E.M.; Zhao, X.G.; Park, J.-Y.; Gu, H.-B. Enhancement of the Photoelectric Performance of Dye-Sensitized Solar Cells Using Ag-doped TiO2 Nanofibers in a TiO2 Film as Electrode. Nanoscale Res. Lett. 2012, 7, 97. [Google Scholar] [CrossRef]
- Shakir, S.; Abd-ur-Rehman, H.M.; Yunus, K.; Iwamoto, M.; Periasamy, V. Fabrication of Un-Doped and Magnesium Doped TiO2 Films by Aerosol Assisted Chemical Vapor Deposition for Dye Sensitized Solar Cells. J. Alloy. Compd. 2018, 737, 740–747. [Google Scholar] [CrossRef]
- Supriyanto, A.; Furqoni, L.; Nurosyid, F.; Hidayat, J.; Suryana, R. Effect of Sintering Temperatures and Screen Printing Types on TiO2 Layers in DSSC Applications. AIP Conf. Proc. 2016, 1717, 040001. [Google Scholar] [CrossRef]
- Noor, S.; Sajjad, S.; Leghari, S.A.K.; Shaheen, S.; Iqbal, A. ZnO/TiO2 Nanocomposite Photoanode as an Effective UV-vis Responsive Dye Sensitized Solar Cell. Mater. Res. Express 2018, 5, 095905. [Google Scholar] [CrossRef]
- Hamadanian, M.; Safaei-Ghomi, J.; Hosseinpour, M.; Masoomi, R.; Jabbari, V. Uses of New Natural Dye Photosensitizers in Fabrication of High Potential Dye-Sensitized Solar Cells (DSSCs). Mater. Sci. Semicond. Process. 2014, 27, 733–739. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Electrochemical Properties of Fiber-in-Tube-and Filled-Structured TiO2 Nanofiber Anode Materials for Lithium-Ion Batteries. Chem.-Eur. J. 2015, 21, 11082–11087. [Google Scholar] [CrossRef]
- Jin, E.M.; Park, J.-Y.; Hwang, K.-J.; Gu, H.-B.; Jeong, S.M. Biotemplated Hybrid TiO2 Nanoparticle and TiO2–SiO2 Nanocomposites for Dye-Sensitized Solar Cells. Mater. Lett. 2014, 131, 190–193. [Google Scholar] [CrossRef]
- Desai, U.V.; Xu, C.; Wu, J.; Gao, D. Hybrid TiO2-SnO2 Nanotube Arrays for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 3232–3239. [Google Scholar] [CrossRef]
- Luan, X.; Wang, Y. Ultrathin Exfoliated TiO2 Nanosheets Modified with ZrO2 for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 18917–18923. [Google Scholar] [CrossRef]
- Jin, E.M.; Park, J.-Y.; Gu, H.-B.; Jeong, S.M. Synthesis of SnO2 Hollow Fiber Using Kapok Biotemplate for Application in Dye-Sensitized Solar Cells. Mater. Lett. 2015, 159, 321–324. [Google Scholar] [CrossRef]
- Swathy, K.S.; Abraham, P.A.; Panicker, N.R.; Pramanik, N.C.; Jacob, K.S. Nanostructured Anatase Titania Spheres as Light Scattering Layer in Dye-Sensitized Solar Cells. Procedia Technol. 2016, 24, 767–773. [Google Scholar] [CrossRef]
- Shabdan, Y.; Ronasi, A.; Coulibaly, P.; Moniruddin, M.; Nuraje, N. Engineered Core-Shell Nanofibers for Electron Transport Study in Dye-Sensitized Solar Cells. AIP Adv. 2017, 7, 065008. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Yu, J.; Hu, Y.; Xia, C.; Zhang, J.; Liu, R. Significant Enhancement of Power Conversion Efficiency for Dye Sensitized Solar Cell Using 1D/3D Network Nanostructures as Photoanodes. Sci. Rep. 2015, 5, 9305. [Google Scholar] [CrossRef]
- Weintraub, B.; Wei, Y.; Wang, Z.L. Optical Fiber/Nanowire Hybrid Structures for Efficient Three-Dimensional Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2009, 121, 9143–9147. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, L.; Mo, L.; Jiang, L.; Hu, L.; Li, Z.; Chen, S.; Dai, S. TiO2 Microspheres with Controllable Surface Area and Porosity for Enhanced Light Harvesting and Electrolyte Diffusion in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2015, 25, 5946–5953. [Google Scholar] [CrossRef]
- Ruqaishy, M.A.; Marzouqi, F.A.; Qi, K.; Liu, S.-Y.; Karthikeyan, S.; Kim, Y.; Al-Kindy, S.M.Z.; Kuvarega, A.T.; Selvaraj, R. Template-Free Preparation of TiO2 Microspheres for the Photocatalytic Degradation of Organic Dye. Korean J. Chem. Eng. 2018, 35, 2283–2289. [Google Scholar] [CrossRef]
- Choi, J.; Yoo, K.S.; Kim, J. Spray Pyrolysis Synthesis of Mesoporous TiO2 Microspheres and Their Post Modification for Improved Photocatalytic Activity. Korean J. Chem. Eng. 2018, 35, 2480–2486. [Google Scholar] [CrossRef]
- Park, N.-G.; Schlichthörl, G.; Van de Lagemaat, J.; Cheong, H.M.; Mascarenhas, A.; Frank, A.J. Dye-Sensitized TiO2 Solar Cells: Structural and Photoelectrochemical Characterization of Nanocrystalline Electrodes Formed from the Hydrolysis of TiCl4. J. Phys. Chem. B 1999, 103, 3308–3314. [Google Scholar] [CrossRef]
- Liu, L.; Niu, H.; Zhang, S.Z.; Wan, L.; Miao, S.; Xu, J. Improved Performance of Dye-Sensitized Solar Cells: An TiO2–Nano-SiO2 Hybrid Photoanode with Post-Treatment of TiCl4 Aqueous Solution. Appl. Surf. Sci. 2012, 261, 8–13. [Google Scholar] [CrossRef]







| Photo-Electrodes | BET (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) | Crystal Structure | Voc (V) | Jsc (mA cm−2) | FF (%) | η (%) |
|---|---|---|---|---|---|---|---|---|
| TNP | 140.08 | 7.13 | 0.2763 | Anatase | 0.69 ± 0.02 | 12.51 ± 0.60 | 53 ± 2 | 4.6 ± 0.07 |
| PTF | 48.01 | 18.47 | 0.2794 | Anatase, Rutile | 0.65 ± 0.01 | 10.30 ± 0.96 | 56 ± 2 | 3.8 ± 0.19 |
| DTF | 23.16 | 5.48 | 0.0460 | Anatase, Rutile | 0.66 ± 0.01 | 10.18 ± 1.24 | 48 ± 7 | 3.2 ± 0.15 |
| TNP-PTF | - | - | - | - | 0.68 ± 0.01 | 14.95 ± 1.03 | 54 ± 2 | 5.4 ± 0.17 |
| TNP-DTF | - | - | - | - | 0.68 ± 0.01 | 13.42 ± 0.50 | 55 ± 1 | 5.1 ± 0.16 |
| TNP/PTF | - | - | - | - | 0.66 ± 0.02 | 16.96 ± 0.79 | 56 ± 2 | 5.9 ± 0.13 |
| TNP/DTF | - | - | - | - | 0.66 ± 0.01 | 14.39 ± 0.50 | 54 ± 1 | 5.2 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, M.S.; Cho, J.S.; Wang, X.L.; Jin, E.M.; Jeong, S.M.; Kang, D.-W. Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers. Nanomaterials 2019, 9, 95. https://doi.org/10.3390/nano9010095
Jo MS, Cho JS, Wang XL, Jin EM, Jeong SM, Kang D-W. Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers. Nanomaterials. 2019; 9(1):95. https://doi.org/10.3390/nano9010095
Chicago/Turabian StyleJo, Min Su, Jung Sang Cho, Xuan Liang Wang, En Mei Jin, Sang Mun Jeong, and Dong-Won Kang. 2019. "Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers" Nanomaterials 9, no. 1: 95. https://doi.org/10.3390/nano9010095