Study of Precipitates in Oxide Dispersion-Strengthened Steels by SANS, TEM, and APT
Abstract
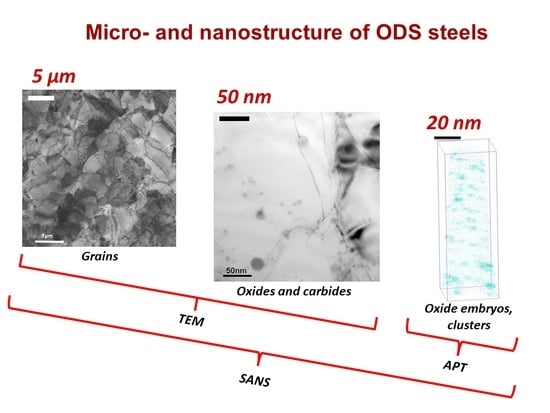
:1. Introduction
2. Materials and Methods
3. Results
3.1. APT and TEM Results
3.2. SANS Results
3.3. Analysis of Hardening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klueh, R.L.; Shingledecker, J.P.; Swindeman, R.W.; Hoelzer, D.T. Oxide Dispersion-Strengthened Steels: A Comparison of Some Commercial and Experimental Alloys. J. Nucl. Mater. 2005, 341, 103–114. [Google Scholar] [CrossRef]
- Beatty, T.G.; Millan, P.P. Progress in the Utilization of an Oxide-Dispersion-Strengthened Alloy for Small Engine Turbine Blades; SAE International: Warrendale, PN, USA, 1984; p. 841512. [Google Scholar] [CrossRef]
- Shigeharu, U.; Toshio, N.; Hirokazu, O. Development of oxide dispersion strengthened ferritic steels for FBR core application. J. Nucl. Sci. Technol. 1996, 34, 256–263. [Google Scholar] [CrossRef]
- Gwon, J.H.; Kim, J.H.; Lee, K.A. Effects of Cryomilling on the Microstructures and High Temperature Mechanical Properties of Oxide Dispersion Strengthened Steel. J. Nucl. Mater. 2015, 459, 205–216. [Google Scholar] [CrossRef]
- Shigeharu, U.; Takanari, O.; Masayuki, F.; Toshimi, K.; Syunji, M.; Hideharu, N. Characterization of high temperature creep properties in recrystallized 12Cr–ODS ferritic steel claddings. J. Nucl. Sci. Technol. 2001, 39, 872–879. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Shalaev, V.I.; Arbuzov, V.L.; Goshchitskii, B.N.; Tian, Y.; Qun, W.; Jiguang, S. Radiation Resistance and Thermal Creep of ODS Ferritic Steels. J. Nucl. Mater. 2001, 295, 265–272. [Google Scholar] [CrossRef]
- Ukai, S.; Fujiwara, M. Perspective of ODS Alloys Application in Nuclear Environments. J. Nucl. Mater. 2002, 307–311, 749–757. [Google Scholar] [CrossRef]
- Lindau, R.; Möslang, A.; Rieth, M.; Klimiankou, M.; Materna-Morris, E.; Alamo, A.; Tavassoli, A.F.; Cayron, C.; Lancha, A.M.; Fernandez, P.; et al. Present development status of EUROFER and ODS-EUROFER for application in blanket concepts. Fus. Eng. Des. 2005, 75–79, 989–996. [Google Scholar] [CrossRef]
- Carlan, Y.; Bechade, J.-L.; Dubuisson, P.; Seran, J.-L.; Billot, P.; Bougault, A.; Cozzika, T.; Doriot, S.; Hamon, D.; Henry, J.; et al. CEA developments of new ferritic ODS alloys for nuclear applications. J. Nucl. Mater. 2009, 386–388, 430–432. [Google Scholar] [CrossRef]
- Mateus, R.; Carvalho, P.A.; Nunes, D.; Alves, L.C.; Franco, N.; Correia, J.B.; Alves, E. Microstructural characterization of the ODS Eurofer 97 EU-batch. Fus. Eng. Des. 2011, 86, 2386–2389. [Google Scholar] [CrossRef]
- Kimura, A.; Cho, H.-S.; Toda, N.; Kasada, R.; Yutani, K.; Kishimoto, H.; Iwata, N.; Ukai, S.; Fujiwara, M. High Burnup Fuel Cladding Materials R&D for Advanced Nuclear Systems. J. Nucl. Sci. Technol. 2007, 44, 323–328. [Google Scholar] [CrossRef]
- Dubuisson, P.; Carlan, Y.; Garat, V.; Blat, M. ODS ferritic/martensitic alloys for sodium fast reactor fuel pin cladding. J. Nucl. Mater. 2012, 428, 6–12. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Olier, P.; Carlan, D.; Baluc, N. Development and characterisation of a new ODS ferritic steel for fusion reactor application. J. Nucl. Mater. 2009, 393, 114–119. [Google Scholar] [CrossRef]
- Klimiankou, M.; Lindau, R.; Möslang, A. TEM characterization of structure and composition of nanosized ODS particles in reduced activation ferritic–martensitic steels. J. Nucl. Mater. 2004, 329–333, 347–351. [Google Scholar] [CrossRef]
- Klimenkov, M.; Lindau, R.; Möslang, A. New insights into the structure of ODS particles in the ODS-Eurofer alloy. J. Nucl. Mater. 2009, 386, 553. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Dickerson, P.; Odette, G.R.; Maloy, S.A.; Misra, A.; Nastsi, M. On the structure and chemistry of complex oxide nanofeatures in nanostructured ferritic alloy U14YWT. Philos. Mag 2012, 92, 2089–2107. [Google Scholar] [CrossRef]
- Hsiung, L.; Fluss, M.; Tumey, S.; Kuntz, J.; El-Dasher, B.; Wall, M.; Choi, B.; Kimura, A.; Willaime, F.; Serruys, Y.J. HRTEM study of oxide nanoparticles in K3-ODS ferritic steel developed for radiation tolerance. Nucl. Mater. 2011, 409, 72–79. [Google Scholar] [CrossRef]
- Oono, N.H.; Ukai, S.; Hayashi, S.; Ohtsuka, S.; Kaito, T.; Kimura, A.; Torimaru, T.; Sakamoto, K.J. Growth of oxide particles in FeCrAl- oxide dispersion strengthened steels at high temperature. J. Nucl. Mater 2017, 493, 180–188. [Google Scholar] [CrossRef]
- Song, P.; Kimura, A.; Yabuuchi, K.; Dou, P.; Watanabe, H.; Gao, J.; Huang, Y.J. Assessment of phase stability of oxide particles in different types of 15Cr-ODS ferritic steels under 6.4 MeV Fe ion irradiation at 200 °C. J. Nucl. Mater. 2020, 529, 151953. [Google Scholar] [CrossRef]
- Miller, M.K.; Hoelzer, D.T.; Kenik, E.A.; Russell, K.F. Nanometer scale precipitation in ferritic MA/ODS alloy MA957. J. Nucl. Mater. 2004, 329–333, 338–341. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Russell, K.F.; Heatherly, L.; Hoelzer, D.T.; Maziasz, P.J. Atom probe tomography of nanoscale particles in ODS ferritic alloys. Mater. Sci. Eng. 2003, A353, 140–145. [Google Scholar] [CrossRef]
- Williams, C.A.; Marquis, E.A.; Cerezo, A.; Smith, G.D.W. Nanoscale characterisation of ODS–Eurofer 97 steel: An atom-probe tomography study. J. Nucl. Mater. 2010, 400, 37–45. [Google Scholar] [CrossRef]
- Baluc, N.; Boutard, J.; Dudarev, S.; Rieth, M.; Correia, J.B.; Fournier, B.; Henry, J.; Legendre, F.; Leguey, T.; Lewandowska, M.; et al. Review on the EFDA work programme on nanostructured ODS RAF steels. J. Nucl. Mater. 2011, 417, 149–153. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S.; Kaito, T.; de Carlan, Y.; Ribis, J.; Malaplate, J. Oxide dispersion-strengthened/ferrite-martensite steels as core materials for Generation IV nuclear reactors. Chapter 10. In Structural Materials for Generation IV Nuclear Reactors; Woodhead Publishing: Sawston, UK, 2017; pp. 357–414. [Google Scholar] [CrossRef]
- Odette, G.R.; Cunningham, N.J.; Stan, T.; Alam, M.E.; de Carlan, Y. Nano-Oxide Dispersion-Strengthened Steels. In Structural Alloys for Nuclear Energy Applications; Odette, G.R., Zinkle, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 529–583. [Google Scholar] [CrossRef]
- Ribis, J.; Mouton, I.; Baumier, C.; Gentils, A.; Loyer-Prost, M.; Lunéville, L.; Siméone, D. Nano-structured materials under irradiation: Oxide Dispersion-Strengthened steels. Nanomaterials 2021, 11, 2590. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Khomich, A.A.; Bogachev, A.A.; Nikitin, A.A.; Khoroshilov, V.V.; Lukyanchuk, A.A.; Raznitsyn, O.A.; Shutov, A.S.; Vasiliev, A.L.; Presniakov, M.Y. Comprehensive analysis of nanostructure of oxide dispersion strengthened steels as prospective materials for nuclear reactors. Phys. At. Nucl. 2020, 83, 1425–1433. [Google Scholar] [CrossRef]
- Rogozhkin, S.; Bogachev, A.; Korchuganova, O.; Nikitin, A.; Orlov, N.; Aleev, A.; Zaluzhnyi, A.; Kozodaev, M.; Kulevoy, T.; Chalykh, B.; et al. Nanostructure evolution in ODS steels under ion irradiation. Nucl. Mater. Energy 2016, 9, 66–74. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Bogachev, A.A.; Nikitin, A.A.; Vasiliev, A.L.; Presnyakov, M.Y.; Tomut, M.; Trautmann, C. TEM analysis of radiation effects in ODS steels induced by swift heavy ions. Nucl. Instr. Meth. Phys. Res. B 2021, 486, 1–10. [Google Scholar] [CrossRef]
- Oono, N.; Ukai, S. Precipitation of Oxide Particles in Oxide Dispersion Strengthened (ODS) Ferritic Steels. Mater. Trans. 2018, 59, 1651–1658. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Gorshkova, Y.E.; Bokuchava, G.D.; Khomich, A.A.; Bogachev, A.A.; Nikitin, A.A. Study of Nanostructure of Oxide Dispersion-Strengthened Steels by Small-Angle X-Ray Scattering. Phys. At. Nucl. 2022, 85, 1467–1472. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Materialia 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Coppola, R.; Klimenkov, M.; Lindau, R.; Möslang, A.; Valli, M.; Wiedenmann, A. Recent applications of small-angle neutron scattering in the characterization of irradiated steels for nuclear technologies. J. Nucl. Mater. 2011, 409, 100–105. [Google Scholar] [CrossRef]
- Heintze, C.; Bergner, F.; Ulbricht, A.; Hernández-Mayoral, M.; Keiderling, U.; Lindau, R.; Weissgärber, T. Microstructure of oxide dispersion strengthened Eurofer and iron–chromium alloys investigated by means of small-angle neutron scattering and transmission electron microscopy. J. Nucl. Mater. 2011, 416, 35–39. [Google Scholar] [CrossRef]
- Coppola, R.; Klimenkov, M.; Lindau, R.; Mangiapia, G. Small-angle neutron scattering (SANS) characterization of 13.5 Cr oxide dispersion strengthened ferritic steel for fusion applications. Nucl. Mater. Energy 2020, 24, 100778. [Google Scholar] [CrossRef]
- Mathon, M.H.; Perrut, M.; Zhong, S.Y.; de Carlan, Y. Small angle neutron scattering study of martensitic/ferritic ODS alloys. J. Nucl. Mater. 2012, 428, 147–153. [Google Scholar] [CrossRef]
- Almásy, L. New measurement control software on the Yellow Submarine SANS instrument at the Budapest Neutron Centre. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2021, 15, 527–531. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Teng, H.; Jiang, X.; Xu, H.; Xia, Y.; Luo, H.; Xiao, L.; Tan, Y.; Zhu, L.; et al. Small Angle Neutron Scattering Spectrometer Detector of China Spallation Neutron Source. Nucl. Phys. Rev. 2019, 36, 204–210. [Google Scholar] [CrossRef]
- Ke, Y.; He, C.; Zheng, H.; Geng, Y.; Fu, J.; Zhang, S.; Hu, H.; Wang, S.; Zhou, B.; Wang, F.; et al. The time-of-flight Small-Angle Neutron Spectrometer at China Spallation Neutron Source. Neutron News 2018, 29, 14–17. [Google Scholar] [CrossRef]
- Rogozhkin, S.V.; Aleev, A.A.; Lukyanchuk, A.A.; Shutov, A.S.; Raznitsyn, O.A.; Kirillov, S.E. Atom probe tomography prototype with laser evaporation. Instrum. Exp. Tech. 2017, 60, 428–433. [Google Scholar] [CrossRef]
- Gault, B.; Müller, M.; La Fontaine, A.; Moody, M.P.; Shariq, A.; Cerezo, A.; Ringer, S.P.; Smith, G.D.W. Influence of surface migration on the spatial resolution of pulsed laser atom probe tomography. J. Appl. Phys. 2010, 108, 044904. [Google Scholar] [CrossRef]
- Pereira, V.S.M.; Davis, T.P.; Mayoral, M.H.; Kumar, A.; Schut, H.; Sietsma, J. Investigation of Coarsening of Oxide Nanoparticles at 1400 K and Its Effect on the Microstructure Formation of an ODS Eurofer Steel. Mater. Charact. 2022, 185, 111723. [Google Scholar] [CrossRef]
- Fu, J.; Davis, T.P.; Kumar, A.; Richardson, I.M.; Hermans, M.J.M. Characterisation of the Influence of Vanadium and Tantalum on Yttrium-Based Nano-Oxides in ODS Eurofer Steel. Mater. Charact. 2021, 175, 111072. [Google Scholar] [CrossRef]
- Yu, H.; Wang, H.; Kondo, S.; Okuno, Y.; Kasada, R.; Oono-Hori, N.; Ukai, S. Radiation Tolerance of Alumina Scale Formed on FeCrAl ODS Ferritic Alloy. Nucl. Mater. Energy 2021, 29, 101102. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. TEM and HRTEM Study of Oxide Particles in an Al-Alloyed High-Cr Oxide Dispersion Strengthened Steel with Zr Addition. J. Nucl. Mater. 2014, 444, 441–453. [Google Scholar] [CrossRef]
- Dou, P.; Jiang, S.; Qiu, L.; Kimura, A. Effects of Contents of Al, Zr and Ti on Oxide Particles in Fe–15Cr–2W–0.35Y2O3 ODS Steels. J. Nucl. Mater. 2020, 531, 152025. [Google Scholar] [CrossRef]
- He, P.; Lindau, R.; Moeslang, A.; Sandim, H.R.Z. The Influence of Thermomechanical Processing on the Microstructure and Mechanical Properties of 13.5Cr ODS Steels. Fusion Eng. Des. 2013, 88, 2448–2452. [Google Scholar] [CrossRef]
- He, P.; Liu, T.; Möslang, A.; Lindau, R.; Ziegler, R.; Hoffmann, J.; Kurinskiy, P.; Commin, L.; Vladimirov, P.; Nikitenko, S.; et al. XAFS and TEM Studies of the Structural Evolution of Yttrium-Enriched Oxides in Nanostructured Ferritic Alloys Fabricated by a Powder Metallurgy Process. Mater. Chem. Phys. 2012, 136, 990–998. [Google Scholar] [CrossRef]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Cryst. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Michels, A.; Malyeyev, A.; Titov, I.; Honecker, D.; Cubitt, R.; Blackburn, E.; Suzuki, K. Magnetic Guinier law. IUCrJ 2020, 7, 136–142. [Google Scholar] [CrossRef]
- Muñoz, A.; Monge, M.A.; Savoini, B.; Pareja, R.; Radulescu, A. SANS characterization of particle dispersions in W-Ti and W-V alloys. Int. J. Refract. Met. Hard Mater. 2016, 61, 173–178. [Google Scholar] [CrossRef]
- De Geuser, F.; Deschamps, A. Precipitate characterisation in metallic systems by small-angle X-ray or neutron scattering. C. R. Phys. 2012, 13, 246–256. [Google Scholar] [CrossRef]
- Cousin, F. Small angle neutron scattering. EPJ Web Conf. 2015, 104, 01004. [Google Scholar] [CrossRef]
- Ohnuma, M.; Suzuki, J.; Ohtsuka, S.; Kim, S.-W.; Kaito, T.; Inoue, M.; Kitazawa, H. A New Method for the Quantitative Analysis of the Scale and Composition of Nanosized Oxide in 9Cr-ODS Steel. Acta Mater. 2009, 57, 5571–5581. [Google Scholar] [CrossRef]
- Wiedenmann, A. Small Angle Neutron Scattering Investigations of Magnetic Nanostructures, Chapter 10. In Neutron Scattering from Magnetic Materials; Chatterji, T., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2006; pp. 473–520. [Google Scholar] [CrossRef]
- Swenson, M.J.; Dolph, C.K.; Wharry, J.P. The effects of oxide evolution on mechanical properties in proton- and neutron-irradiated Fe-9%Cr ODS steel. J. Nucl. Mater. 2016, 479, 426–435. [Google Scholar] [CrossRef]
- Gil, E.; Ordás, N.; García-Rosales, C.; Iturriza, I. Microstructural characterization of ODS ferritic steels at different processing stages. Fus. Eng. Des. 2015, 98–99, 1973–1977. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, T.S.; Hoelzer, D.T.; Park, C.H.; Yeom, J.T.; Hong, J.K. Temperature dependence of strengthening mechanisms in the nanostructured ferritic alloy 14YWT: Part II—Mechanistic models and predictions. Mater. Sci. Eng. A 2013, 559, 111–118. [Google Scholar] [CrossRef]
- Mouawad, B.; Boulnat, X.; Fabrègue, D.; Perez, M.; de Carlan, Y. Tailoring the microstructure and the mechanical properties of ultrafine grained high strength ferritic steels by powder metallurgy. J. Nucl. Mater. 2015, 465, 54–62. [Google Scholar] [CrossRef]















| Steel | Al | Ni | Zr | Mn | Cr | W | Y | O | V | C | N | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eurofer ODS | - | 0.02 | - | 0.39 | 9.8 | 0.34 | 0.13 | 0.34 | 0.22 | 0.40 | 0.21 | 0.06 |
| KP-4 ODS | 7.56 | - | 0.19 | - | 15.9 | 0.58 | 0.16 | 0.57 | - | - | - | - |
| 13.5Cr-Fe3Y ODS | - | - | - | - | 14.6 | 0.6 | 0.3 | - | - | - | - |
| Steel | Alloying Type | Thermomechanical Treatment |
|---|---|---|
| Eurofer ODS | Mechanical alloying of metal and Y2O3 powders | Normalized at 1100 °C for 30 min with water quenching, followed by tempering at 750 °C for 2 h with air cooling |
| KP-4 ODS | Mechanical alloying of metal and Y2O3 powders | Encapsulated in a soft steel capsule and degassed in vacuum at 400 °C for 3 h |
| 13.5Cr-Fe3Y ODS | Yttrium was introduced through the Fe3Y intermetallic powder, and oxygen was introduced through the oxidized powder of the matrix steel | Degassed for 4 h at 400 °C, then subjected to hot isostatic pressing at 1150 °C for 2 h at 100 MPa |
| Material | Phase | Chemical Composition/Formula | Average Size | Number Density, 1022 m−3 | Δρ2 (SLD) Nuclear, 1021 | Δρ2 (SLD) Magnetic, 1021 | A |
|---|---|---|---|---|---|---|---|
| Eurofer ODS | Matrix | Fe89 Cr10 Mn0.4 V0.2 N0.1 O0.1 W0.3 Y0.1 | - | - | - | - | - |
| Grains | - | 0.5 ± 0.2 μm | - | - | - | - | |
| Oxides | Y2O3 | 6 ± 2 nm | 4 ± 1 | 0.98 | 2.3 | 3.3 | |
| Clusters | Fe46 Cr20 V9 O11 Y10 W0.3 Mn0.5 N0.1 | 2 ± 1 nm | 32 ± 5 | 0.43 | 0.24 | 1.6 | |
| 13.5Cr-Fe3Y ODS | Matrix | Fe85 Cr13 Mn0.7 O0.2 V0.1 Y0.4 Ni0.2 Si0.1 W0.4 | - | - | - | - | - |
| Grains | - | 0.8 ± 0.4 μm | - | - | - | - | |
| Oxides | Y2O3 | 6 ± 1 nm | 0.8 ± 0.2 | 0.9 | 2.2 | 3.4 | |
| Clusters | Fe50 Cr14 Mn0.5 O6 Y11 Ni0.6 Si0.2 W0.11 | 2 ± 1 nm | 32 ± 4 | 0.5 | 0.27 | 1.5 | |
| KP-4 ODS | Matrix | Fe76 Cr16 O0,4 Y0,1 Al7 Zr0,2 W0,6 | - | - | - | - | - |
| Grains | - | 0.6 ±0.4 μm | - | - | - | - | |
| Oxides | Y4Zr3O12 | 5 ± 2 nm | 2 ± 1 | 0.9 | 2.2 | 3.4 | |
| Clusters | Fe49 Cr12 O19 Y12 Al5 Zr3 | 3 ± 1 nm | 10 ± 2 | 0.5 | 0.27 | 1.5 |
| Steel | TEM Oxide Size, nm | 2Rg (No Field) 1/2*, nm | 2Rg (1.1 T Field) 2*, nm | |
|---|---|---|---|---|
| Eurofer ODS | ~40 | 46/35 | (||H) | 39 |
| (⊥H) | 37 | |||
| 13.5Cr-Fe3Y ODS | ~50 | 50/40 | (||H) | 45 |
| (⊥H) | 43 | |||
| KP-4 ODS | ~50 | 54/46 | (||H) | 46 |
| (⊥H) | 43 | |||
| Steel | TEM | APT | SANS (No Field) 1/2* | SANS (1.1 T Field) 2* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d, nm | N, 1022 m−3 | d, nm | N, 1022 m−3 | Object Type | d, nm | N, 1022 m−3 | Azimuthal Angle | d, nm | N, 1022 m−3 | |
| Eurofer ODS | 6 ± 2 | 4 ± 1 | 2 ± 1 | 30 ± 5 | Oxide | 4/6 | 5/1.7 | (||H) | 6 | 3.3 |
| (⊥H) | 1.2 | |||||||||
| Cluster | 2/3 | 40/25 | (||H) | 2 | 42 | |||||
| (⊥H) | 35 | |||||||||
| 13.5Cr-Fe3Y ODS | 6 ± 1 | 0.8 ± 0.2 | 2 ± 1 | 32 ± 4 | Oxide | 5/7 | 3.5/0.5 | (||H) | 6 | 3.2 |
| (⊥H) | 1.1 | |||||||||
| Cluster | 2/2 | 29/27 | (||H) | 2 | 37 | |||||
| (⊥H) | 28 | |||||||||
| KP-4 ODS | 5 ± 2 | 2 ± 1 | 3 ± 1 | 9 ± 2 | Oxide | 7/7 | 2/1.6 | (||H) | 6 | 1.2 |
| (⊥H) | 0.8 | |||||||||
| Cluster | 3/3 | 6/4.6 | (||H) | 2 | 11.3 | |||||
| (⊥H) | 6.2 | |||||||||
| Steel | TEM and APT, GPa | SANS (No Field) 1/2*, GPa | SANS (1.1 T, ||H) 2*, GPa | SANS (1.1 T, ⊥H) 2*, GPa | Microindentation, Hv, GPa |
|---|---|---|---|---|---|
| Eurofer ODS | 4.2 ± 0.2 | 4.1 ± 0.2/3.6 ± 0.2 | 4.1 ± 0.2 | 3.3 ± 0.2 | 3.8 ± 0.2 |
| 13.5Cr-Fe3Y ODS | 2.9 ± 0.2 | 3.6 ± 0.2/2.7 ± 0.3 | 3.7 ± 0.2 | 3.0 ± 0.3 | 3.0 ± 0.2 |
| KP-4 ODS | 3.3 ± 0.2 | 3.5 ± 0.2/3.4 ± 0.2 | 3.1 ± 0.3 | 2.9 ± 0.3 | 3.2 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozhkin, S.V.; Klauz, A.V.; Ke, Y.; Almásy, L.; Nikitin, A.A.; Khomich, A.A.; Bogachev, A.A.; Gorshkova, Y.E.; Bokuchava, G.D.; Kopitsa, G.P.; et al. Study of Precipitates in Oxide Dispersion-Strengthened Steels by SANS, TEM, and APT. Nanomaterials 2024, 14, 194. https://doi.org/10.3390/nano14020194
Rogozhkin SV, Klauz AV, Ke Y, Almásy L, Nikitin AA, Khomich AA, Bogachev AA, Gorshkova YE, Bokuchava GD, Kopitsa GP, et al. Study of Precipitates in Oxide Dispersion-Strengthened Steels by SANS, TEM, and APT. Nanomaterials. 2024; 14(2):194. https://doi.org/10.3390/nano14020194
Chicago/Turabian StyleRogozhkin, Sergey V., Artem V. Klauz, Yubin Ke, László Almásy, Alexander A. Nikitin, Artem A. Khomich, Aleksei A. Bogachev, Yulia E. Gorshkova, Gizo D. Bokuchava, Gennadiy P. Kopitsa, and et al. 2024. "Study of Precipitates in Oxide Dispersion-Strengthened Steels by SANS, TEM, and APT" Nanomaterials 14, no. 2: 194. https://doi.org/10.3390/nano14020194