Influence of the Al-Doped ZnO Sputter-Deposition Temperature on Cu(In,Ga)Se2 Solar Cell Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin-Film Deposition
2.2. Characterization
3. Results
3.1. Composition
3.2. Crystallographic Properties
3.3. Morphological Properties
3.4. Optical Properties
3.5. Chemical States
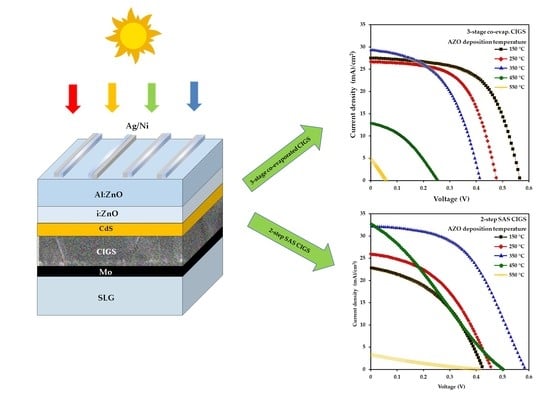
3.6. Device Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nakamura, M.; Yamaguchi, K.; Kimoto, Y.; Yasaki, Y.; Kato, T.; Sugimoto, H. Cd-Free Cu(In,Ga)(Se,S)2 Thin-Film Solar Cell With Record Efficiency of 23.35%. IEEE J. Photovolt. 2019, 9, 1863–1867. [Google Scholar] [CrossRef]
- Global Solar. Available online: http://globalsolar.com/ (accessed on 29 January 2022).
- Ramanujam, J.; Singh, U.P. Copper Indium Gallium Selenide Based Solar Cells—A Review. Energy Environ. Sci. 2017, 10, 1306–1319. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Ho, S.-J.; Chen, G.-H.; Yeh, C.-W.; Chen, P.-R.; Chen, H.-S. Toward Low-Cost Large-Area CIGS Thin Film III: Effect of Se Concentration on Crystal Growth and Defect Formation of Sequentially Electrodeposited CIGS Thin Films. Solar Energy 2016, 132, 547–557. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of Heavy Alkali Elements in Cu(In,Ga)Se2 Solar Cells with Efficiencies up to 22.6%. Phys. Status Solidi RRL 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Luo, S.; Lee, J.-H.; Liu, C.-W.; Shieh, J.-M.; Shen, C.-H.; Wu, T.-T.; Jang, D.; Greer, J.R. Strength, Stiffness, and Microstructure of Cu(In,Ga)Se2 Thin Films Deposited via Sputtering and Co-Evaporation. Appl. Phys. Lett. 2014, 105, 011907. [Google Scholar] [CrossRef]
- Langhorst, M.; Bykov, E.; Jiang, Q.; Kim, J.; Rozeveld, S.; Mushrush, M.; Wall, A.; Khare, A.; Feist, R. Control of CIGS Roughness by Initial Selenization Temperature. In Proceedings of the IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, K.; Yun, J.H.; Ahn, B.T. A New Simple Route to Grow Cu(In,Ga)Se2 Thin Films with Large Grains in the Co-Evaporation Process. Curr. Appl. Phys. 2018, 18, 912–918. [Google Scholar] [CrossRef]
- Aberle, A.G. Thin-Film Solar Cells. Thin Solid Films 2009, 517, 4706–4710. [Google Scholar] [CrossRef]
- Lee, S.; Bang, S.; Park, J.; Park, S.; Ko, Y.; Jeon, H. AZO/Au/AZO Multilayer as a Transparent Conductive Electrode. Phys. Status Solidi A 2012, 209, 698–701. [Google Scholar] [CrossRef]
- Sutthana, S.; Hongsith, N.; Choopun, S. AZO/Ag/AZO Multilayer Films Prepared by DC Magnetron Sputtering for Dye-Sensitized Solar Cell Application. Curr. Appl. Phys. 2010, 10, 813–816. [Google Scholar] [CrossRef]
- Miao, D.; Jiang, S.; Shang, S.; Chen, Z. Highly Transparent and Infrared Reflective AZO/Ag/AZO Multilayer Film Prepared on PET Substrate by RF Magnetron Sputtering. Vacuum 2014, 106, 1–4. [Google Scholar] [CrossRef]
- Wang, T.; Diao, X.; Ding, P. Thermal Stability of Electrical Properties of ZnO:Al Films Deposited by Room Temperature Magnetron Sputtering. J. Alloys Compd. 2011, 509, 4910–4915. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, J.B.; Han, J.G. Optical Absorption Spectroscopy of Facing Targets and Conventional Magnetron Sputtering during Process of Al-Doped ZnO Films. Surf. Coat. Technol. 2014, 254, 371–375. [Google Scholar] [CrossRef]
- Misra, P.; Mandati, S.; Rao, T.N.; Sarada, B.V. A Multi-Layer Cu:Ga/In Sputtered Precursor to Improve Structural Properties of CIGS Absorber Layer. Mater. Today Proc. 2021, 39, 2037–2041. [Google Scholar] [CrossRef]
- Perrenoud, J.; Kranz, L.; Buecheler, S.; Pianezzi, F.; Tiwari, A.N. The Use of Aluminium Doped ZnO as Transparent Conductive Oxide for CdS/CdTe Solar Cells. Thin Solid Films 2011, 519, 7444–7448. [Google Scholar] [CrossRef]
- Reddy, V.R.M.; Cho, H.; Gedi, S.; Reddy, K.T.R.; Kim, W.K.; Park, C. Effect of Sulfurization Temperature on the Efficiency of SnS Solar Cells Fabricated by Sulfurization of Sputtered Tin Precursor Layers Using Effusion Cell Evaporation. J. Alloys Compd. 2019, 806, 410–417. [Google Scholar] [CrossRef]
- Lee, D.; Cho, J.Y.; Heo, J. Improved Efficiency of Sb2Se3/CdS Thin-Film Solar Cells: The Effect of Low-Temperature Pre-Annealing of the Absorbers. Solar Energy 2018, 173, 1073–1079. [Google Scholar] [CrossRef]
- Theuring, M.; Vehse, M.; von Maydell, K.; Agert, C. AZO-Ag-AZO Transparent Electrode for Amorphous Silicon Solar Cells. Thin Solid Films 2014, 558, 294–297. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Chen, J.; Fang, L.; Wang, L. Structural and Optoelectronic Properties of AZO Thin Films Prepared by RF Magnetron Sputtering at Room Temperature. Trans. Nonferrous Met. Soc. China 2016, 26, 1655–1662. [Google Scholar] [CrossRef]
- Zhu, H.; Hüpkes, J.; Bunte, E.; Huang, S.M. Oxygen Influence on Sputtered High Rate ZnO:Al Films from Dual Rotatable Ceramic Targets. Appl. Surf. Sci. 2010, 256, 4601–4605. [Google Scholar] [CrossRef]
- Rana, V.S.; Rajput, J.K.; Pathak, T.K.; Pal, P.K.; Purohit, L.P. Impact of RF Sputtering Power on AZO Thin Films for Flexible Electro-Optical Applications. Cryst. Res. Technol. 2021, 56, 2000144. [Google Scholar] [CrossRef]
- Zhou, H.B.; Zhang, H.Y.; Tan, M.L.; Zhang, W.J.; Zhang, W.L. Effects of Sputtering Pressure on Properties of Al Doped ZnO Thin Films Dynamically Deposited by Rf Magnetron Sputtering. Mater. Res. 2012, 16, 390–394. [Google Scholar] [CrossRef]
- Patel, K.H.; Rawal, S.K. Influence of Power and Temperature on Properties of Sputtered AZO Films. Thin Solid Films 2016, 620, 182–187. [Google Scholar] [CrossRef]
- Subramanyam, T.K.; Goutham, P.; Pavan Kumar, S.; Yadhuraj, S.R.; Geetha, K.S. Optimization of Sputtered AZO Thin Films for Device Application. Mater. Today Proc. 2018, 5, 10851–10859. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, H.; Zhang, W.; Laing, X.; Guo, Y.; Li, Z.; Chen, J.; Xu, Y.; Mai, Y. Control of Oxygen for Magnetron Sputtered ZnO:Al Window Layer in Cu(In,Ga)Se2 Thin Film Solar Cells. Phys. Status Solidi A 2017, 214, 1700132. [Google Scholar] [CrossRef]
- Sung, J.-C.; Lu, C.-H. Effects of Sputtering Conditions on the Photovoltaic Properties of Al-Doped Zinc Oxide Films for Cu(In,Ga)Se2 Thin-Film Solar Cells. J. Mater. Sci. Mater. Electron. 2017, 28, 15442–15450. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, R. Optimization of Al-Doped ZnO Films by RF Magnetron Sputtering at Room Temperature for Cu (In, Ga) Se2 Solar Cells. J. Phys. Conf. Ser. 2020, 1549, 042006. [Google Scholar] [CrossRef]
- Chang, J.-C.; Guo, J.-W.; Hsieh, T.-P.; Yang, M.-R.; Chiou, D.-W.; Cheng, H.-T.; Yeh, C.-L.; Li, C.-C.; Chu, S.-Y. Effects of Substrate Temperature on the Properties of Transparent Conducting AZO Thin Films and CIGS Solar Cells. Surf. Coat. Technol. 2013, 231, 573–577. [Google Scholar] [CrossRef]
- Noikaew, B.; Chatraphorn, S. Effects of Substrate Temperatures in the Three–Stage Growth of CuIn1−xGaxSe2 Thin Films and Their Photovoltaic Performances. Surf. Coat. Technol. 2016, 307, 547–553. [Google Scholar] [CrossRef]
- Park, Y.; Ferblantier, G.; Slaoui, A.; Dinia, A.; Park, H.; Alhammadi, S.; Kim, W.K. Yb-Doped Zinc Tin Oxide Thin Film and Its Application to Cu(InGa)Se2 Solar Cells. J. Alloys Compd. 2020, 815, 152360. [Google Scholar] [CrossRef]
- Alhammadi, S.; Moon, K.; Park, H.; Kim, W.K. Effect of Different Cadmium Salts on the Properties of Chemical-Bath-Deposited CdS Thin Films and Cu(InGa)Se2 Solar Cells. Thin Solid Films 2017, 625, 56–61. [Google Scholar] [CrossRef]
- Jseng, Y.-Y.; Chao, C.-J.; Sung, H.-H.; Chen, T.-C. CIGS Thin Film and Device Performance Produced through a Variation Ga Concentration during Three-Stage Growth Process. Mater. Sci. Semicond. Process. 2018, 87, 162–166. [Google Scholar] [CrossRef]
- Koo, J.; Kwon, S.; Roh, Y.-S.; Lee, S.-J.; Jung, K.-Y.; Shafarman, W.N.; Park, J.-H.; Kim, D.H.; Myoung, J.-M.; Kim, W.K. Effect of Reaction Temperature and Time during Two-Step Selenization and Sulfurization of Se-Coated CuGa/In Precursors. Electron. Mater. Lett. 2016, 12, 484–493. [Google Scholar] [CrossRef]
- Park, J.; Kim, W.K. Effect of Sputtering Conditions of Co-Sputtered Cu–In–Ga Precursors on Cu(InGa)Se2 Photovoltaic Absorber Formation. Thin Solid Films 2014, 572, 61–67. [Google Scholar] [CrossRef]
- Li, B.-Y.; Zhang, Y.; Wang, H.; Wang, B.; Wu, L.; Sun, Y. Preferred Orientation of Cu(In,Ga)Se2 Thin Film Deposited on Stainless Steel Substrate. Prog. Photovolt. Res. Appl. 2012, 21, 838–848. [Google Scholar] [CrossRef]
- Chaisitsak, S.; Yamada, A.; Konagai, M. Preferred Orientation Control of Cu(In1-xGax)Se2(x≈0.28) Thin Films and Its Influence on Solar Cell Characteristics. Jpn. J. Appl. Phys. 2002, 41, 507–513. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, F.; Liu, L.; Yu, Z.; Zhang, Y.; Zhao, Y. Control over the Preferred Orientation of CIGS Films Deposited by Magnetron Sputtering Using a Wetting Layer. Electron. Mater. Lett. 2016, 12, 59–66. [Google Scholar] [CrossRef]
- Shin, D.H.; Shin, Y.M.; Kim, J.H.; Ahn, B.T.; Yoon, K.H. Control of the Preferred Orientation of Cu(In,Ga)Se2 Thin Film by the Surface Modification of Mo Film. J. Electrochem. Soc. 2011, 159, B1–B5. [Google Scholar] [CrossRef]
- Londhe, P.U.; Rohom, A.B.; Fernandes, R.; Kothari, D.C.; Chaure, N.B. Development of Superstrate CuInGaSe2 Thin Film Solar Cells with Low-Cost Electrochemical Route from Nonaqueous Bath. ACS Sustain. Chem. Eng. 2018, 6, 4987–4995. [Google Scholar] [CrossRef]
- Huang, P.C.; Sung, C.C.; Chen, J.H.; Hsiao, R.C.; Hsu, C.Y. Effect of Selenization and Sulfurization on the Structure and Performance of CIGS Solar Cell. J. Mater. Sci. Mater. Electron. 2017, 29, 1444–1450. [Google Scholar] [CrossRef]
- Moon, K.; Kim, W.K. Cu(InGa)Se2 Absorber Formation by in-Situ, Low-Temperature Annealing of Co-Evaporated Bilayer (InGa)2Se3/CuSe Precursors. Thin Solid Films 2015, 596, 63–67. [Google Scholar] [CrossRef]
- He, B.; Xu, J.; Xing, H.; Wang, C.; Zhang, X. The Effect of Substrate Temperature on High Quality C-Axis Oriented AZO Thin Films Prepared by DC Reactive Magnetron Sputtering for Photoelectric Device Applications. Superlattices Microstruct. 2013, 64, 319–330. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, J. Silver-Doped ZnO for Photocatalytic Degradation of Methylene Blue. Korean J. Chem. Eng. 2020, 37, 1226–1232. [Google Scholar] [CrossRef]
- Jahagirdar, A.H.; Kadam, A.A.; Dhere, N.G. Role of i-ZnO in optimizing open circuit voltage of CIGS2 and CIGS thin film solar cells. In Proceedings of the IEEE 4th World Conference on Photovoltaic Energy Conference (WCPEC–4), Waikoloa, HI, USA, 7–12 May 2006. [Google Scholar] [CrossRef]
- Kuang, X.-P.; Zhang, H.-Y.; Wang, G.-G.; Cui, L.; Zhu, C.; Jin, L.; Sun, R.; Han, J.-C. Effect of Deposition Temperature on the Microstructure and Surface Morphology of C-Axis Oriented AlN Films Deposited on Sapphire Substrate by RF Reactive Magnetron Sputtering. Superlattices Microstruct. 2012, 52, 931–940. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, Y.; Zhu, S.; Ding, J.; Zhang, D.; Yin, A.; Chen, P. Substrate Temperature Dependent Properties of Sputtered AlN:Er Thin Film for In-Situ Luminescence Sensing of Al/AlN Multilayer Coating Health. Materials 2018, 11, 2196. [Google Scholar] [CrossRef]
- Kumar, M. Effect of Substrate Temperature on Surface Morphology and Optical Properties of Sputter Deposited Nanocrystalline Nickel Oxide Films. Mater. Res. Express 2019, 6, 096404. [Google Scholar] [CrossRef]
- Huang, L.; Li, B.; Ren, N. Enhancing Optical and Electrical Properties of Al-Doped ZnO Coated Polyethylene Terephthalate Substrates by Laser Annealing Using Overlap Rate Controlling Strategy. Ceram. Int. 2016, 42, 7246–7252. [Google Scholar] [CrossRef]
- Li, B.; Yang, G.; Huang, L.; Zu, W.; Li, H.; Wang, Y.; Li, S.; Ren, N. Surface Morphology and Photoelectric Properties of FTO Ceramic Thin Films under a Simple Transparent Cover-Assisted Laser Annealing. Mater. Res. Bull. 2018, 108, 151–155. [Google Scholar] [CrossRef]
- Winfield, R.J.; Koh, L.H.K.; O’Brien, S.; Crean, G.M. Excimer Laser Processing of ZnO Thin Films Prepared by the Sol–Gel Process. Appl. Surf. Sci. 2007, 254, 855–858. [Google Scholar] [CrossRef]
- Suchea, M.; Christoulakis, S.; Katsarakis, N.; Kitsopoulos, T.; Kiriakidis, G. Comparative Study of Zinc Oxide and Aluminum Doped Zinc Oxide Transparent Thin Films Grown by Direct Current Magnetron Sputtering. Thin Solid Films 2007, 515, 6562–6566. [Google Scholar] [CrossRef]
- Prabhakar, T.; Dai, L.; Zhang, L.; Yang, R.; Li, L.; Guo, T.; Yan, Y. Effects of Growth Process on the Optical and Electrical Properties in Al-Doped ZnO Thin Films. J. Appl. Phys. 2014, 115, 083702. [Google Scholar] [CrossRef]
- Challali, F.; Mendil, D.; Touam, T.; Chauveau, T.; Bockelée, V.; Sanchez, A.G.; Chelouche, A.; Besland, M.-P. Effect of RF Sputtering Power and Vacuum Annealing on the Properties of AZO Thin Films Prepared from Ceramic Target in Confocal Configuration. Mater. Sci. Semicond. Process. 2020, 118, 105217. [Google Scholar] [CrossRef]
- Park, J.-H.; Pak, H.-K.; Cho, C.-R. Deposition-Temperature Effects on AZO Thin Films Prepared by RF Magnetron Sputtering and Their Physical Properties. J. Korean Phys. Soc. 2006, 49, 584–588. [Google Scholar]
- Lu, C.J.; Kuang, A.X.; Huang, G.Y.; Wang, S.M. XPS Study on Composition and Structure of Epitaxial KTa1−xNbxO3 (KTN) Thin Films Prepared by the Sol-Gel Process. J. Mater. Sci. 1996, 31, 3081–3085. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green Approach for Hierarchical Nanostructured Ag-ZnO and Their Photocatalytic Performance under Sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Al Farsi, B.; Souier, T.M.; Al Marzouqi, F.; Al Maashani, M.; Bououdina, M.; Widatallah, H.M.; Al Abri, M. Structural and Optical Properties of Visible Active Photocatalytic Al Doped ZnO Nanostructured Thin Films Prepared by Dip Coating. Opt. Mater. 2021, 113, 110868. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I.; Perveen, I.; Ahmed, S. Superior Dye Degradation Using SnO2-ZnO Hybrid Heterostructure Catalysts. Korean J. Chem. Eng. 2018, 36, 56–62. [Google Scholar] [CrossRef]
- Li, Y.-C.; Jin, F.-L.; Park, S.-J. Oxygen-Vacancy-Rich Spinel CoFe2O4 Nanocrystals Anchored on Cage-like Carbon for High-Performance Oxygen Electrocatalysis. Korean J. Chem. Eng. 2021, 38, 2134–2140. [Google Scholar] [CrossRef]
- Li, L.; Fang, L.; Zhou, X.J.; Liu, Z.Y.; Zhao, L.; Jiang, S. X-ray Photoelectron Spectroscopy Study and Thermoelectric Properties of Al-Doped ZnO Thin Films. J. Electron Spectrosc. Relat. Phenom. 2009, 173, 7–11. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Nakada, T.; Kunioka, A. Improved Jsc in CIGS Thin Film Solar Cells Using a Transparent Conducting ZnO:B Window Layer. Sol. Energy Mater. Sol. Cells 2001, 67, 267–271. [Google Scholar] [CrossRef]
- Cho, D.-H.; Chung, Y.-D.; Lee, K.-S.; Park, N.-M.; Kim, K.-H.; Choi, H.-W.; Kim, J. Influence of Growth Temperature of Transparent Conducting Oxide Layer on Cu(In,Ga)Se2 Thin-Film Solar Cells. Thin Solid Films 2012, 520, 2115–2118. [Google Scholar] [CrossRef]
- Werner, F.; Babbe, F.; Burkhart, J.; Spindler, C.; Elanzeery, H.; Siebentritt, S. Interdiffusion and Doping Gradients at the Buffer/Absorber Interface in Thin-Film Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 28553–28565. [Google Scholar] [CrossRef] [PubMed]
- Koprek, A.; Zabierowski, P.; Pawlowski, M.; Sharma, L.; Freysoldt, C.; Gault, B.; Wuerz, R.; Cojocaru-Mirédin, O. Effect of Cd Diffusion on the Electrical Properties of the Cu(In,Ga)Se2 Thin-Film Solar Cell. Sol. Energy Mater. Sol. Cells 2021, 224, 110989. [Google Scholar] [CrossRef]
- Kijima, S.; Nakada, T. High-Temperature Degradation Mechanism of Cu(In,Ga)Se2-Based Thin Film Solar Cells. Appl. Phys. Express 2008, 1, 075002. [Google Scholar] [CrossRef]
- Nakada, T. Nano-Structural Investigations on Cd-Doping into Cu(In,Ga)Se2 Thin Films by Chemical Bath Deposition Process. Thin Solid Films 2000, 361, 346–352. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.; Al-Ammar, E.A.; Kwon, H.; Ahn, B.T. Effects of Zn Diffusion from (Zn,Mg)O Buffer to CIGS Film on the Performance of Cd-Free Cu(In,Ga)Se2 Solar Cells. ECS J. Solid State Sci. Technol. 2014, 3, Q99–Q103. [Google Scholar] [CrossRef]
- Nakada, T.; Kunioka, A. Direct Evidence of Cd Diffusion into Cu(In,Ga)Se2 Thin Films during Chemical-Bath Deposition Process of CdS Films. Appl. Phys. Lett. 1999, 74, 2444–2446. [Google Scholar] [CrossRef]












| CIGS Process | Atomic Composition | Atomic Ratios | ||||
|---|---|---|---|---|---|---|
| Cu | In | Ga | Se | Cu/III | Ga/III | |
| 3-Stage co-evaporation | 0.22 | 0.19 | 0.06 | 0.53 | 0.86 | 0.24 |
| 2-Step SAS | 0.26 | 0.2 | 0.07 | 0.46 | 0.93 | 0.25 |
| Growth Process of CIGS | AZO Substrate Temperature (°C) | Solar Cell Performance Parameters | |||
|---|---|---|---|---|---|
| JSC (mA/cm2) | VOC (V) | FF (%) | Efficiency (%) | ||
| 3-stage co-evaporation | 150 | 27.57 | 0.57 | 60.33 | 9.43 |
| 250 | 26.77 | 0.48 | 59.04 | 7.55 | |
| 350 | 29.46 | 0.41 | 50.20 | 6.14 | |
| 450 | 28.62 | 0.25 | 37.87 | 2.75 | |
| 550 | 4.85 | 0.057 | 26.29 | 0.07 | |
| 2-step SAS | 150 | 22.85 | 0.43 | 43.23 | 4.21 |
| 250 | 25.92 | 0.46 | 43.90 | 5.22 | |
| 350 | 32.28 | 0.59 | 50.15 | 9.51 | |
| 450 | 32.67 | 0.50 | 27.07 | 4.45 | |
| 550 | 3.30 | 0.414 | 19.92 | 0.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Alhammadi, S.; Minnam Reddy, V.R.; Park, C.; Kim, W.K. Influence of the Al-Doped ZnO Sputter-Deposition Temperature on Cu(In,Ga)Se2 Solar Cell Performance. Nanomaterials 2022, 12, 3326. https://doi.org/10.3390/nano12193326
Park H, Alhammadi S, Minnam Reddy VR, Park C, Kim WK. Influence of the Al-Doped ZnO Sputter-Deposition Temperature on Cu(In,Ga)Se2 Solar Cell Performance. Nanomaterials. 2022; 12(19):3326. https://doi.org/10.3390/nano12193326
Chicago/Turabian StylePark, Hyeonwook, Salh Alhammadi, Vasudeva Reddy Minnam Reddy, Chinho Park, and Woo Kyoung Kim. 2022. "Influence of the Al-Doped ZnO Sputter-Deposition Temperature on Cu(In,Ga)Se2 Solar Cell Performance" Nanomaterials 12, no. 19: 3326. https://doi.org/10.3390/nano12193326