Flexible Humidity Sensors Based on Multidimensional Titanium Dioxide/Cellulose Nanocrystals Composite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cellulose Nanocrystals and Titanium Dioxide Nanoparticles
2.2. Fabrication of Humidity Sensors
2.3. Material Characterization
2.4. Humidity Sensing Test
2.5. Calculation Method
3. Results and Discussion
3.1. Morphological and Structural Characteristics
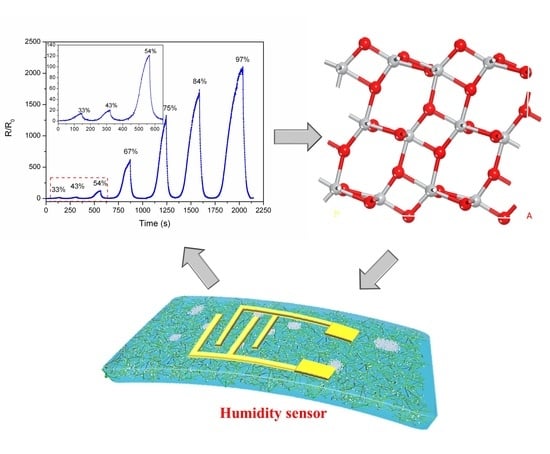
3.2. Humidity Sensing Characteristics
3.3. Density Functional Theory Analysis and Sensing Mechanism of TiO2/CNC-Based Humidity Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A. Organic thin-film capacitive and resistive humidity sensors: A focus review. Adv. Mater. Interfaces 2018, 5, 1800969. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.E.; Kim, D.H.; Shim, Y.S.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly selective and sensitive chemoresistive humidity sensors based on rGO/MoS2 Van der Waals composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/biopolymer composite systems for humidity sensor applications: A review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G. Nanocoil-based fast-response and flexible humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Wang, L.; Tian, M.; Zhang, Y.; Sun, F.; Qu, L. Helical core-sheath elastic yarn-based dual strain/humidity sensors with mxene sensing layer. J. Mater. Sci. 2020, 55, 6187–6194. [Google Scholar] [CrossRef]
- Li, B.; Xiao, G.; Liu, F.; Qiao, Y.; Li, C.; Lu, Z. A flexible humidity sensor based on silk fabrics for human respiration monitoring. J. Mater. Chem. C 2018, 6, 4549–4554. [Google Scholar] [CrossRef]
- Yu, S.; Chen, C.; Zhang, H.; Zhang, J.; Liu, J. Design of high sensitivity graphite carbon nitride/zinc oxide humidity sensor for breath detection. Sens. Actuators B 2021, 332, 129536. [Google Scholar] [CrossRef]
- Tachibana, S.; Wang, Y.; Sekine, T.; Yoshida, A.; Takeda, Y.; Abe, M.; Miura, R.; Watanabe, Y.; Kumaki, D.; Tokito, S. Flexible printed temperature sensor with high humidity stability using bilayer passivation. Flex. Print. Electron. 2021, 6, 034002. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Wang, G.; Li, G.; Zhao, D.; Sun, N.; Li, F.; Zhang, H.; Han, J.; Yang, Y. A flexible ultra-sensitive triboelectric tactile sensor of wrinkled PDMS/MXene composite films for E-skin. Nano Energy 2021, 81, 105663. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohyd. Polym. 2021, 270, 118385. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal based composites: A review. Compos. Part C 2021, 5, 100164. [Google Scholar] [CrossRef]
- Sun, Y.; Chu, Y.; Wu, W.; Xiao, H. Nanocellulose-based lightweight porous materials: A review. Carbohyd. Polym. 2021, 255, 117489. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Farzaneh, A.; Mohammadzadeh, A.; Esrafili, M.D.; Mermer, O. Experimental and theoretical study of TiO2 based nanostructured semiconducting humidity sensor. Ceram. Int. 2019, 45, 8362–8369. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Devi, K.S. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Farzaneh, A.; Esrafili, M.D.; Mermer, Ö. Development of TiO2 nanofibers based semiconducting humidity sensor: Adsorption kinetics and DFT computations. Mater. Chem. Phys. 2020, 239, 121981. [Google Scholar] [CrossRef]
- Zheng, M.; Jia, C.; Sharman, E.; Jiang, J.; Fan, W.; Zhao, X. Maximizing the synergistic effect of PdAu catalysts on TiO2 (101) for robust CO2 reduction: A DFT study. Appl. Surf. Sci. 2021, 563, 150365. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.F.; Medina, F.; González, S. DFT study of methylene blue adsorption on ZnTiO3 and TiO2 surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, W.; Li, Y. Computational study of surface orientation effect of rutile TiO2 on H2S and CO sensing mechanism. Appl. Surf. Sci. 2019, 495, 143619. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Wang, X.; Liao, J.; Xia, H.; Akbar, S.A.; Li, J.; Lin, S.; Li, X.; Wang, J. Hierarchical structured TiO2 nano-tubes for formaldehyde sensing. Ceram. Int. 2012, 38, 6341–6347. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocompositefilms. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Abiaziem, C.V.; Williams, A.B.; Inegbenebor, A.I.; Onwordi, C.T.; EhiEromosele, C.O.; Petrik, L.F. Preparation and characterisation of cellulose nanocrystal from sugarcane peels by XRD, SEM and CP/MAS 13C NMR. J. Phys. Conf. Ser. 2019, 1299, 012123. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Y.; Wang, J. Novel polyvinyl alcohol (PVA)/cellulose nanocrystal (CNC) supramolecular composite hydrogels: Preparation and application as soil conditioners. Nanomaterials 2019, 9, 1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trandaflović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasinska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B 2017, 203, 740–752. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, J.; Zhan, C.; Kong, F.; Li, W.; Yang, C.; Hsiao, B.S. Facile synthesis of TiO2/CNC nanocomposites for enhanced Cr(VI) photoreduction: Synergistic roles of cellulose nanocrystals. Carbohyd. Polym. 2020, 233, 115838. [Google Scholar] [CrossRef]
- Dubourg, G.; Segkos, A.; Katona, J.; Radović, M.; Savić, S.; Niarchos, G.; Christos, T.; Crnojević-Bengin, V. Fabrication and characterization of flexible and miniaturized humidity sensors using screen-printed TiO2 nanoparticles as sensitive layer. Sensors 2017, 17, 1854. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Shen, D.; Zou, G.; Wu, A.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-powered, flexible and remote-controlled breath monitor based on TiO2 nanowire networks. Nanotechnology 2019, 30, 325503. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Lee, D. Highly stable and sensitive resistive flexible humidity sensors by means of roll-to-roll printed electrodes and flower-like TiO2 nanostructures. Ceram. Int. 2019, 45, 985–992. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, M.; Xiao, Y.; Zou, G.; Hu, L.; Zhao, B.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-powered, rapid-response, and highly flexible humidity sensors based on moisture-dependent voltage generation. ACS Appl. Mater. Interfaces 2019, 11, 14249–14255. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cai, J.; Tao, X.; Tan, M. Comp. First-principles study of H2S adsorption and dissociation on Mo (110). Mater. Sci. 2015, 101, 47–55. [Google Scholar]
- He, Y.; Tilocca, A.; Dulub, O.; Selloni, A.; Diebold, U. Local ordering and electronic signatures of subsmonolayer water on anatase TiO2 (101). Nat. Mater. 2009, 8, 585–589. [Google Scholar] [CrossRef]
- Landmann, M.; Rauls, E.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. Condens. Matter 2012, 24, 195503. [Google Scholar] [CrossRef] [Green Version]
- Wendt, S.; Schaub, R.; Matthiesen, J.; Vestergaard, E.K.; Wahlström, E.; Rasmussen, M.D.; Thostrup, P.; Molina, L.M.; Lægsgaard, E.; Stensgaard, I.; et al. Oxygen vacancies on TiO2 (110) and their interaction with H2O and O2: A combined high-resolution STM and DFT study. Surf. Sci. 2005, 598, 226–245. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y. Interplay between water and TiO2 anatase (101) surface with subsurface oxygen vacancy. Phys. Rev. Lett. 2014, 112, 206101.1–206101.5. [Google Scholar] [CrossRef] [Green Version]
- Kumari, L.; Kumar, U.; Sinha, L.; Prasad, O.; Yadav, B.C.; Gupta, M. Surface modification and characterization of h-BN-doped PVP thin film and its application as humidity sensor with theoretical DFT calculations. Chem. Pap. 2021, 75, 4055–4068. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Zhou, C.; Xiao, Y.; Meng, B.; Wang, Z.; Huang, D.; Xing, C.; Peng, Z. High-performance humidity sensor based on urchin-like composite of Ti3C2 MXene-derived TiO2 nanowires. ACS Appl. Mater. Interfaces 2019, 11, 38116–38125. [Google Scholar] [CrossRef]
- Gupta, S.P.; Pawbake, A.S.; Sathe, B.R.; Late, D.J.; Walke, P.S. Superior humidity sensor and photodetector of mesoporous ZnO nanosheets at room temperature. Sens. Actuators B 2019, 293, 83–92. [Google Scholar] [CrossRef]










| Material | Humidity Range (% RH) | Response (R0/R) | Response/Recovery Time | Substrate | References |
|---|---|---|---|---|---|
| TiO2 | 0–70 | 10 | 3 min/50 s | PET * | [29] |
| TiO2 nanowire network | 30–75 | - | 3.6 s/14 s | PET * | [30] |
| TiO2 nanoflower | 20–95 | 103~104 | hundreds of second | PI * | [31] |
| TiO2 nanowire | 20–90 | - | 4.5 s/2.8 s | ITO * | [32] |
| TiO2 nanoparticles | 11–97 | 9.7-1900 | 34 s/18 s | CNC | This work |
| Adsorption Site | Eads(eV) | Da(Å) | RO-H1 (Å) | RO-H2 (Å) | ∠H-O-H (°) | Band Gap (eV) |
|---|---|---|---|---|---|---|
| Ti5c | −0.693 | 2.313 | 0.984 | 0.980 | 105.103 | 2.491 |
| Ti6c | −0.403 | 2.009 | 0.976 | 3.594 | 78.150 | 2.507 |
| O2c | −0.603 | 2.786 | 0.979 | 0.979 | 106.205 | 2.511 |
| O3c | −0.690 | 2.896 | 0.983 | 0.981 | 104.537 | 2.484 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Wang, H.; Ding, H.; Li, J.; Zhao, H.; Lin, Z.; Xi, H.; Zhang, X. Flexible Humidity Sensors Based on Multidimensional Titanium Dioxide/Cellulose Nanocrystals Composite Film. Nanomaterials 2022, 12, 1970. https://doi.org/10.3390/nano12121970
Tong X, Wang H, Ding H, Li J, Zhao H, Lin Z, Xi H, Zhang X. Flexible Humidity Sensors Based on Multidimensional Titanium Dioxide/Cellulose Nanocrystals Composite Film. Nanomaterials. 2022; 12(12):1970. https://doi.org/10.3390/nano12121970
Chicago/Turabian StyleTong, Xin, Hong Wang, Huiyang Ding, Jing Li, Huifang Zhao, Zhaoyun Lin, Hongxia Xi, and Xuejin Zhang. 2022. "Flexible Humidity Sensors Based on Multidimensional Titanium Dioxide/Cellulose Nanocrystals Composite Film" Nanomaterials 12, no. 12: 1970. https://doi.org/10.3390/nano12121970