Comparative Preparation Method and Associated Cost of Lignin–Cellulose Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
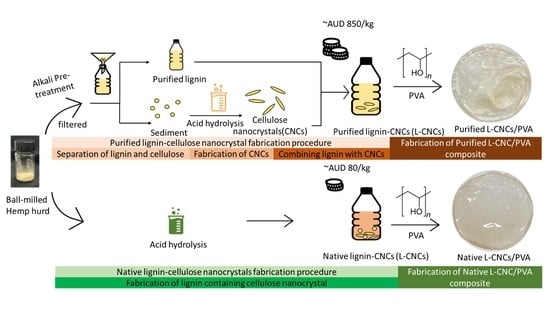
2.2. Preparation of Native and Purified Lignin–Cellulose Nanocrystals (L–CNCs)
2.3. Preparation of L–CNC/PVA Film
2.4. Characterisations
2.4.1. Morphology
2.4.2. Chemical Structure
2.4.3. Absorbance and Transmittance
2.4.4. Tensile Properties
2.4.5. Water-Vapour Properties
2.4.6. Statistical Analyses
3. Results and Discussion
3.1. Morphology
3.2. Absorption Properties
3.3. Chemical Structure
3.4. Tensile Properties
3.5. Water-Vapour Properties
3.6. Cost Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light blocker-A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen performance of lignin from different technical resources and their general synergistic effect with synthetic sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Restrepo, A.; El Mehdi, N.; Boumghar, Y. Quantification and Variability Analysis of Lignin Optical Properties for Colour-Dependent Industrial Applications. Molecules 2018, 23, 377. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-M.; Wang, B.; Yuan, T.-Q.; Zheng, L.; Shi, Q.; Wang, S.-F.; Song, G.-Y.; Sun, R.-C. Tunable, UV-shielding and biodegradable composites based on well-characterized lignins and poly(butylene adipate-co-terephthalate). Green Chem. 2020, 22, 8623–8632. [Google Scholar] [CrossRef]
- Wei, L.; Agarwal, U.P.; Matuana, L.; Sabo, R.C.; Stark, N.M. Performance of high lignin content cellulose nanocrystals in poly(lactic acid). Polymer 2018, 135, 305–313. [Google Scholar] [CrossRef]
- Teh, K.C.; Foo, M.L.; Ooi, C.W.; Leng Chew, I.M. Sustainable and cost-effective approach for the synthesis of lignin-containing cellulose nanocrystals from oil palm empty fruit bunch. Chemosphere 2021, 267, 129277. [Google Scholar] [CrossRef]
- Wimalasiri, E.M.; Jahanshiri, E.; Chimonyo, V.G.P.; Kuruppuarachchi, N.; Suhairi, T.A.S.T.M.; Azam-Ali, S.N.; Gregory, P.J. A framework for the development of hemp (Cannabis sativa L.) as a crop for the future in tropical environments. Ind. Crops Prod. 2021, 172, 113999. [Google Scholar] [CrossRef]
- Heitzmann, M.; Ali, A.; Legras, A.; Vandi, L.-J.; Milne, J. Hemp hurd flour as an alternative low cost filler in wood plastic composites. In Proceedings of the International Conference on Performance-Based and Life-Cycle Structural Engineering, Brisbane, Australia, 9–11 December 2015; pp. 109–115. [Google Scholar] [CrossRef] [Green Version]
- Luzi, F.; Fortunati, E.; Puglia, D.; Lavorgna, M.; Santulli, C.; Kenny, J.M.; Torre, L. Optimized extraction of cellulose nanocrystals from pristine and carded hemp fibres. Ind. Crops Prod. 2014, 56, 175–186. [Google Scholar] [CrossRef]
- Vyas, P.; Kumar, A.; Singh, S. Biomass breakdown: A review on pretreatment, instrumentations and methods. Front. Biosci. Elit. 2018, 10, 155–174. [Google Scholar] [CrossRef] [Green Version]
- Monteil-Rivera, F.; Phuong, M.; Ye, M.; Halasz, A.; Hawari, J. Isolation and characterization of herbaceous lignins for applications in biomaterials. Ind. Crops Prod. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Tyagi, P.; Gutierrez, J.N.; Nathani, V.; Lucia, L.A.; Rojas, O.J.; Hubbe, M.A.; Pal, L. Hydrothermal and mechanically generated hemp hurd nanofibers for sustainable barrier coatings/films. Ind. Crops Prod. 2021, 168, 113582. [Google Scholar] [CrossRef]
- Zhang, Y.; Haque, A.N.M.A.; Naebe, M. Lignin-Cellulose Nanocrystals from Hemp Hurd as Light-Coloured Ultraviolet (UV) Functional Filler for Enhanced Performance of Polyvinyl Alcohol Nanocomposite Films. Nanomaterials 2021, 11, 3425. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Browne, C.; Raghuwanshi, V.S.; Mouterde, L.M.M.; Simon, G.P.; Allais, F.; Garnier, G. Phenolic ester-decorated cellulose nanocrystals as UV-absorbing nanoreinforcements in polyvinyl alcohol films. ACS Sustain. Chem. Eng. 2021, 9, 6427–6437. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Biodegradable cotton gin trash/poly(vinyl alcohol) composite plastic: Effect of particle size on physicochemical properties. Powder Technol. 2020, 375, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Remadevi, R.; Hinestroza, J.P.; Wang, X.; Naebe, M. Transparent ultraviolet (UV)-shielding films made from waste hemp hurd and polyvinyl alcohol (PVA). Polymers 2020, 12, 1190. [Google Scholar] [CrossRef]
- Zhu, M.; Song, J.; Li, T.; Gong, A.; Wang, Y.; Dai, J.; Yao, Y.; Luo, W.; Henderson, D.; Hu, L.; et al. Highly Anisotropic, Highly Transparent Wood Composites. Adv. Mater. 2016, 28, 5181–5187. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess. 2019, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Wu, Q.; Young, T.M.; Huang, B.; Wang, S.; Li, Y. Analyzing three-dimensional structure and geometrical shape of individual cellulose nanocrystal from switchgrass. Polym. Compos. 2017, 38, 2368–2377. [Google Scholar] [CrossRef]
- Water Vapor Transmission Rate of Paper and Paperboard at High Temperature and Humidity, Test Method. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T464.aspx (accessed on 11 March 2020).
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinson-Bagby, K.L.; Roberts, R.; Foster, E.J. Effective cellulose nanocrystal imaging using transmission electron microscopy. Carbohydr. Polym. 2018, 186, 429–438. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Nyman, V.; Rose, G.; Ralston, J. The colloidal behaviour of kraft lignin and lignosulfonates. Colloids Surf. 1986, 21, 125–147. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. The effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M.; Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products through Kraft Lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Xu, G.; Ren, S.; Wang, D.; Sun, L.; Fang, G. Fabrication and Properties of Alkaline Lignin/Poly (Vinyl Alcohol) Blend Membranes. BioResources 2013, 8, 2510–2520. [Google Scholar] [CrossRef] [Green Version]
- Degen, I.A. Detection of the Methoxyl Group by Infrared Spectroscopy. Appl. Spectrosc. 2016, 22, 164–166. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Physicochemical properties of film fabricated from cotton gin trash. Mater. Chem. Phys. 2020, 239, 122009. [Google Scholar] [CrossRef]
- Huzyan, H.I.; Aziz, A.A.; Hussin, M.H. Ecofriendly wood adhesives from date palm fronds lignin for plywood. BioResources 2021, 16, 4106–4125. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Naebe, M. Flexible water-resistant semi-transparent cotton gin trash/poly (vinyl alcohol) bio-plastic for packaging application: Effect of plasticisers on physicochemical properties. J. Clean. Prod. 2021, 303, 126983. [Google Scholar] [CrossRef]
- Chetouani, A.; Elkolli, M.; Bounekhel, M.; Benachour, D. Chitosan/oxidized pectin/PVA blend film: Mechanical and biological properties. Polym. Bull. 2017, 74, 4297–4310. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dhepe, P.L. Solid base catalyzed depolymerization of lignin into low molecular weight products. Green Chem. 2017, 19, 778–788. [Google Scholar] [CrossRef]
- Montes, M.L.I.; Luzi, F.; Dominici, F.; Torre, L.; Cyras, V.P.; Manfredi, L.B.; Puglia, D. Design and Characterization of PLA Bilayer Films Containing Lignin and Cellulose Nanostructures in Combination with Umbelliferone as Active Ingredient. Front. Chem. 2019, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Marchi, B.C.; Keten, S. Microstructure and Size Effects on the Mechanics of Two Dimensional, High Aspect Ratio Nanoparticle Assemblies. Front. Mater. 2019, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, A.; Warsi, M.F.; Sarwar, M.I.; Ishaq, M. Improvement in tensile properties of PVC–montmorillonite nanocomposites through controlled uniaxial stretching. Bull. Mater. Sci. 2012, 35, 539–544. [Google Scholar] [CrossRef]
- Rahman, W.A.; Sudin, S.N.A.; Din, S.N. Physical and mechanical properties of Pandanus amaryllifolius fiber reinforced low density polyethylene composite for packaging application. In Proceedings of the 2012 IEEE Symposium on Humanities, Science and Engineering Research, Kuala Lumpur, Malaysia, 24–27 June 2012; pp. 345–349. [Google Scholar] [CrossRef]
- Designerdata Polyvinyl Chloride Hard. Available online: https://designerdata.nl/materials/plastics/thermo-plastics/polyvinyl-chloride-hard (accessed on 30 September 2021).
- Haque, A.N.M.A.; Naebe, M. Sustainable biodegradable denim waste composites for potential single-use packaging. Sci. Total Environ. 2022, 809, 152239. [Google Scholar] [CrossRef]
- Jared Mullane Electricity Costs Per kWh|QLD, SA, VIC, NSW Rates–Canstar Blue. Available online: https://www.canstarblue.com.au/solar-power/electricity-costs-kwh/ (accessed on 1 October 2021).







| Length (nm) | Height (nm) | Aspect Ratio | Yield (%) | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| Purified L–CNCs | 465.3 ± 159.5 | 27.99 ± 2.68 | 16.6 | 117.5 | −7.6 ± 5.5 |
| Native L–CNCs | 485.3 ± 172.3 | 16.18 ± 1.365 | 30.0 | 13.8 | −24.5 ± 4.5 |
| T280nm% * | T400nm% | T(UVA)% | T(UVB)% | Normalised UPF | |
|---|---|---|---|---|---|
| Pure PVA | 76.02 ± 3.65 | 91.41 ± 0.02 | 91.41 ± 0.74 | 87.24 ± 2.83 | 32.82 ± 0.77 |
| Native L–CNCs/PVA | 41.17 ± 4.58 | 82.17 ± 0.95 | 62.83 ± 3.43 | 41.11 ± 4.31 | 50.38 ± 4.94 |
| Purified L–CNCs/PVA | 26.44 ± 15.86 | 55.89 ± 19.03 | 31.14 ± 13.41 | 23.90 ± 11.60 | 42.34 ± 23.42 |
| Purified L–CNCs | Native L–CNCs | |||||||
|---|---|---|---|---|---|---|---|---|
| Electricity * | kW | h | kW-h | Cost AUD/kg | kW | h | kW-h | Cost AUD/kg |
| Mechanical milling | ||||||||
| Cutting | 5 | 0.05 | 0.25 | 0.05 | 5 | 0.05 | 0.25 | 0.05 |
| Attrition | 2.24 | 20 | 44.8 | 8.72 | 2.24 | 20 | 44.8 | 8.72 |
| Magnetic stir | 0.6 | 1 | 0.6 | 0.12 | 0.6 | 7 | 4.2 | 0.82 |
| Centrifuge | 1.05 | 1 | 1.05 | 0.20 | 1.05 | 1 | 1.05 | 0.20 |
| (a) Total cost for electricity | 9.09 | 9.73 | ||||||
| Chemicals for L–CNCs | ||||||||
| NaOH | 18.72 | |||||||
| Na2SO3 | 814.98 | |||||||
| H2SO4 | 8.52 | 72.54 | ||||||
| (b) Total cost for chemicals | 842.22 | 72.54 | ||||||
| (a + b) Total cost for L–CNCs | 851.31 | 82.27 | ||||||
| Composite films | ||||||||
| PVA | 132.53 | 132.53 | ||||||
| L–CNCs (5 wt.%) | 42.57 | 4.11 | ||||||
| Total cost for 5 wt.% film (per/kg) | 166.75 | 130.13 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Haque, A.N.M.A.; Naebe, M. Comparative Preparation Method and Associated Cost of Lignin–Cellulose Nanocrystals. Nanomaterials 2022, 12, 1320. https://doi.org/10.3390/nano12081320
Zhang Y, Haque ANMA, Naebe M. Comparative Preparation Method and Associated Cost of Lignin–Cellulose Nanocrystals. Nanomaterials. 2022; 12(8):1320. https://doi.org/10.3390/nano12081320
Chicago/Turabian StyleZhang, Yi, Abu Naser Md Ahsanul Haque, and Maryam Naebe. 2022. "Comparative Preparation Method and Associated Cost of Lignin–Cellulose Nanocrystals" Nanomaterials 12, no. 8: 1320. https://doi.org/10.3390/nano12081320