Chitin Nanofibril-Nanolignin Complexes as Carriers of Functional Molecules for Skin Contact Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
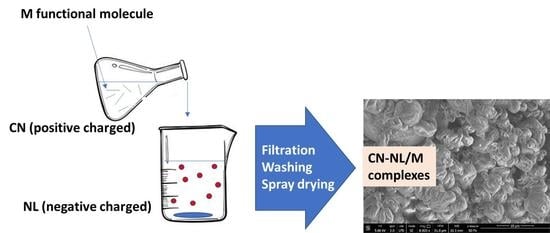
2.2. Morphological Characterization of CN, NL, and CN-NL Complexes
2.3. Chemical Structure and Thermal Stability Charachterisation of CN, NL, and CN-NL Complexes
2.4. In Vitro Culture of HaCaT
2.5. MTT Assay
2.6. Anti-Inflammatory and Immune Responses Evaluation of HaCat Cells
3. Results and Discussion
3.1. Structure, Thermal Stability, and Morphology of CN-NL Spray-Dried Complexes
3.2. Structure and Thermal Stability of CN-NL/M
3.3. HaCaT Cell Viability, and Anti-Inflammatory and Immune Responses of HaCaT Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable bio-based materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Danti, S.; Trombi, L.; Morganti, P.; Donnarumma, G.; Baroni, A.; Fusco, A.; Lazzeri, A. Preparation of Innovative Skin Compatible Films to Release Polysaccharides for Bio-based Beauty Masks. Cosmetics 2018, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Yudin, V.E.; Morganti, G.; Coltelli, M.-B. Trends in Surgical and Beauty Masks for a Cleaner Environment. Cosmetics 2020, 7, 68. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, H.; Jang, M.; Kim, H.; Shin, G.; Koo, J.M.; Lee, M.; Sung, H.K.; Eom, Y.; Yang, H. Biodegradable, efficient, and breathable multi-use face mask filter. Adv. Sci. 2021, 8, 2003155. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef]
- Hartmann, F.; Baumgartner, M.; Kaltenbrunner, M. Becoming Sustainable, The New Frontier in Soft Robotics. Adv. Mater. 2021, 33, 2004413. [Google Scholar] [CrossRef]
- Coiai, S.; Campanella, B.; Paulert, R.; Cicogna, F.; Bramanti, E.; Lazzeri, A.; Pistelli, L.; Coltelli, M.-B. Rosmarinic Acid and Ulvan from Terrestrial and Marine Sources in Anti-Microbial Bionanosystems and Biomaterials. Appl. Sci. 2021, 11, 9249. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Silva, T.G.; Coutinho, H.D.M.; Amina, B.; et al. Antioxidant, Antimicrobial, and Anticancer Effects of Anacardium Plants: An Ethnopharmacological Perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Toan, N. Van Production of chitin and chitosan from partially autolyzed shrimp shell materials. Open Biomater. J. 2009, 1, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, C.; Morganti, P. Preparation of Chitin and Derivatives Thereof for Cosmetic and Therapeutic Use. U.S. Patent 8383157 B2, 26 February 2013. [Google Scholar]
- Vandecasteele, B.; Amery, F.; Ommeslag, S.; Vanhoutte, K.; Visser, R.; Robbens, J.; De Tender, C.; Debode, J. Chemically versus thermally processed brown shrimp shells or Chinese mitten crab as a source of chitin, nutrients or salts and as microbial stimulant in soilless strawberry cultivation. Sci. Total Environ. 2021, 771, 145263. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of Chitin Nanofibers from Mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Hahn, T.; Tafi, E.; von Seggern, N.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; et al. Purification of Chitin from Pupal Exuviae of the Black Soldier Fly. Waste Biomass Valoriz. 2021, 13, 1993–2008. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Su, W.-H.; Chen, H.-C.; Pan, C.-L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.L.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, P.; Morganti, G.; Coltelli, M.-B. Smart and Sustainable Hair Products Based on Chitin-Derived Compounds. Cosmetics 2021, 8, 20. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Jayakumara, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Naira, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Deng, J.; Sun, S.-F.; Zhu, E.-Q.; Yang, J.; Yang, H.-Y.; Wang, D.-W.; Ma, M.-G.; Shi, Z.-J. Sub-micro and nano-lignin materials: Small size and rapid progress. Ind. Crops Prod. 2021, 164, 113412. [Google Scholar] [CrossRef]
- Yang, I.; Zheng, A.; Zhao, Z.; Xia, S.; Wang, Q.; Wei, G.; Huang, Z.; Jiang, L.; Wang, S.; Li, H. Maximizing production of sugar and ultrafine lignin particles from recalcitrant softwood by different acids-assisted organosolvolysis and fast pyrolysis. J. Clean. Prod. 2020, 276, 122827. [Google Scholar] [CrossRef]
- Mili, M.; Hashmi, S.A.R.; Tilwari, A.; Rathore, S.K.S.; Naik, A.; Srivastava, A.K.; Verma, S. Preparation of Nanolignin rich fraction from Bamboo Stem via Green Technology: Assessment of its Antioxidant, Antibacterial and UV Blocking Properties. Environ. Technol. 2021, 1–33. [Google Scholar] [CrossRef]
- Lin, Q.; Yan, Y.; Liu, X.; He, B.; Wang, X.; Wang, X.; Liu, C.; Ren, J. Production of Xylooligosaccharide, Nanolignin, and Nanocellulose through a Fractionation Strategy of Corncob for Biomass Valorization. Ind. Eng. Chem. Res. 2020, 59, 17429–17439. [Google Scholar] [CrossRef]
- Juikar, S.J.; Vigneshwaran, N. Microbial production of coconut fiber nanolignin for application onto cotton and linen fabrics to impart multifunctional properties. Surf. Interfaces 2017, 9, 147–153. [Google Scholar] [CrossRef]
- Morganti, P. Method of Preparation of Chitin and Active Principles Complexes and the So Obtained Complexes. Patent WO2012143875A1, 19 April 2011. [Google Scholar]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panariello, L.; Vannozzi, A.; Morganti, P.; Coltelli, M.-B.; Lazzeri, A. Bio-based and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E. Cosmetics 2021, 8, 27. [Google Scholar] [CrossRef]
- Teno, J.; Pardo-Figuerez, M.; Hummel, N.; Bonin, V.; Fusco, A.; Ricci, C.; Donnarumma, G.; Coltelli, M.-B.; Danti, S.; Lagaron, J.M. Preliminary Studies on an Innovative Bioactive Skin Soluble Beauty Mask Made by Combining Electrospinning and Dry Powder Impregnation. Cosmetics 2020, 7, 96. [Google Scholar] [CrossRef]
- Morganti, P.; Del Ciotto, P.; Gao, X.H. Skin Delivery and Controlled Release of Active Ingredients Nanoencapsulated. Cosmet. Sci. Technol. 2012, 20, 37–42. [Google Scholar]
- Keen, M.A.; Hassan, I. Vitamin E in Dermatology. Indian Derm. J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Scherer Santos, J.; Diniz Tavares, G.; Nogueira Barradas, T. Vitamin E and Derivatives in Skin Health Promotion. In Vitamin E in Health and Disease-Interactions, Diseases and Health Aspects; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Azevedo Martins, T.E.; Sales de Oliveira Pinto, C.A.; Costa de Oliveira, A.; Robles Velasco, M.V.; Gorriti Guitiérrez, A.R.; Cosquillo Rafael, M.F.; Tarazona, J.P.H.; Retuerto-Figueroa, M.G. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Sci. Pharm. 2020, 88, 27. [Google Scholar] [CrossRef]
- Nachbar, F.; Korting, H.C. The role of vitamin E in normal and damaged skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef]
- Telang, P.S. Vitamin C in Dermatology. Indian Dermatol. J. 2013, 4, 143–146. [Google Scholar] [CrossRef]
- De Phillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; La Prade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Costa Caritá, A.; Fonseca-Santos, B.; Daniela Shultz, J.; Michniak-Kohn, B.; Chorilli, M.; Ricci Leonardi, G. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability, Nanomedicine. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 9, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Mitri, K. Carotenoid lutein: A promising candidate for pharmaceutical and nutraceutical applications. J. Diet. Suppl. 2012, 9, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza and its Bioactives: Update and review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Nacanulli, D.; Francini Lesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinjc acid, the active principle of liquerice can reduce the thickness of subcutaneous thigh fat through topical applications. Steroids 2005, 70, 538–542. [Google Scholar] [CrossRef]
- Obama, K.; Kawada-Matsuo, M.; Oogai, Y.; Hayashi, T.; Nakamura, N.; Komatsuzawa, H. Antibacterial effects of glycyrrhetinic acid and its derivatives on Staphilococcus aureus. PLoS ONE 2016, 11, 20165831. [Google Scholar] [CrossRef] [Green Version]
- Wohlrab, J.; Kreft, D. Niacinamide-Mechanism of Action and its Topical use in Dermatology. Ski. Pharmacol. Physiol. 2014, 27, 311–315. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Yi, J.M.; Hyun, J.W. Protects Skin Cells from Oxidative Stress Induced by Particulate Matter. Biomol. Ther. 2019, 27, 562–569. [Google Scholar] [CrossRef]
- Gajbhiyl, S.; Sakharwade, S. Silver Nanoparticles in Cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical Applications: Synthesis, performance and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar]
- Anniboletti, A.; Palombo, M.; Moroni, S.; Bruno, A.; Palombo, P.; Morganti, P. Clinical Activity of Innovative Non-Woven Tissues. In Bionanotechnology to Save the Environment. Plant and Fishery’s Biomass as Alternative to Petrol; Morganti, P., Ed.; MDPI: Basel, Switzerland, 2018; pp. 340–360. [Google Scholar]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S.A. Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Coiai, S.; Cicogna, F.; Pinna, S.; Spiniello, R.; Onor, M.; Oberhauser, W.; Coltelli, M.B.; Passaglia, E. Antibacterial LDPE-based nanocomposites with salicylic and rosmarinic acid-modified layered double hydroxides. Appl. Clay Sci. 2021, 214, 106276. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Muzzarelli, C. Spray-Dried Chitin Nanofibrils, Method for Production and Uses Thereof. Patent WO2007060628A1, 23 November 2006. [Google Scholar]
- Delmas, G.-H.; Benjelloun-Mlayah, B.; Le Bigot, Y.; Delmas, M. Functionality of wheat straw lignin extracted in organic acid media. J. Appl. Polym. Sci. 2011, 121, 491–501. [Google Scholar] [CrossRef]
- Brooker, A.D.M.; Vaccaro, M.; Scialla, S.; Walker, S.J.; Morganti, P.; Carezzi, F.; Benjelloun-Mlayah, B.; Crestini, C.; Lange, H.; Bartzoka, E. A Consumer Goods Product Comprising Chitin Nanofibrils, Lignin and a Polymer or Co-Polymer. Patent EP2995321B1, 31 March 2015. [Google Scholar]
- Morganti, P. Suspensions and Materials Comprising Complexes of Chitin Nanofibrils with Metals. Patent WO2015049656A3, 2 October 2014. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Stoller, M.; Chianese, A. Antibacterial and Anti-inflammatory Green Nanocomposites. Chem. Eng. Trans. 2016, 47, 61–66. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 1, 55–63. [Google Scholar] [CrossRef]
- Fusco, A.; Coretti, L.; Savio, V.; Buommino, E.; Lembo, F.; Donnarumma, G. Biofilm formation and immunomodulatory activity of Proteus mirabilis clinically isolated strains. Int. J. Mol. Sci. 2017, 18, 414. [Google Scholar] [CrossRef] [Green Version]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progr. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Pearson, F.G.; Marchessault, R.H.; Liang, C.Y. Infrared spectra of crystalline polysaccharides. V. Chitin. J. Polym. Sci. 1960, 43, 101–116. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Osada, M.; Miura, C.; Nakagawa, Y.S.; Kaihara, M.; Nikaido, M.; Totani, K. Effects of supercritical water and mechanochemical grinding treatments on physicochemical properties of chitin. Carbohydr. Polym. 2013, 92, 1573–1578. [Google Scholar] [CrossRef]
- Vrandečić, N.S.; Erceg, M.; Jakić, M.; Klarić, I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Zhu, I.; Tsend-Ayush, A.; Yuan, Z.; Wen, J.; Cai, J.; Luo, S.; Yao, J.; Bian, J.; Yin, L.; Zhou, J.; et al. Glycyrrhetinic acid-modified TPGS polymeric micelles for hepatocellular carcinoma-targeted therapy. Int. J. Pharm. 2017, 529, 451–464. [Google Scholar] [CrossRef]
- Silva, S.D.; Rosa, N.F.; Ferreira, A.E.; Boas, L.V.; Bronze, M.R. Rapid Determination of α-Tocopherol in Vegetable Oils by Fourier Transform Infrared Spectroscopy. Food Anal. Methods 2009, 2, 120. [Google Scholar] [CrossRef] [Green Version]
- Bunaciu, A.A.; Bacalum, E.; Aboul-Enein, H.Y.; Udristioiu, G.E.; Fleschin, S. FT-IR Spectrophotometric Analysis of Ascorbic Acid and Biotin and their Pharmaceutical Formulations. Anal. Lett. 2009, 42, 1321–1327. [Google Scholar] [CrossRef]
- Ramalingam, S.; Periandy, S.; Govindarajan, M.; Mohan, S. FT-IR and FT-Raman vibrational spectra and molecular structure investigation of nicotinamide: A combined experimental and theoretical study, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.D.; Abeyratne, C.; Bangamuwa, O.M.; Ludowyke, N.; Dahanayake, D.; Gunasekara, S.; de Silva, N.; de Silva, R.M.; Nalin de Silva, K.M. In-situ formation of supramolecular aggregates between chitin nanofibers and silver nanoparticles. Carbohydr. Polym. 2017, 173, 295–304. [Google Scholar] [CrossRef]
- Jingyana, S.; Yuwen, L.; Zhiyong, W.; Cunxin, W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. Anal. 2013, 77, 116–119. [Google Scholar] [CrossRef]
- Gigante, V.; Panariello, L.; Coltelli, M.-B.; Danti, S.; Obisesan, K.A.; Hadrich, A.; Staebler, A.; Chierici, S.; Canesi, I.; Lazzeri, A.; et al. Liquid and Solid Functional Bio-Based Coatings. Polymers 2021, 13, 3640. [Google Scholar] [CrossRef]
- Chou, C.-W.; Hsu, S.-H.; Chang, H.; Tseng, S.-M.; Lin, H.-R. Enhanced thermal and mechanical properties and biostability of polyurethane containing silver nanoparticles. Polym. Degrad. Stab. 2006, 91, 1017–1024. [Google Scholar] [CrossRef]
- Chatterjee, U.; Jewrajka, S.K.; Guha, S. Dispersion of functionalized silver nanoparticles in polymer matrices: Stability, characterization, and physical properties. Polym. Compos. 2009, 30, 827–834. [Google Scholar] [CrossRef]
- Rhim, W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997, 8, 253–265. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential Involvement of Interleukin-8 (IL-8) in Acute Inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Savio, V.; Donniacuo, M.; Perfetto, B.; Donnarumma, G. Antimicrobial Peptides Human Beta-Defensin-2 and -3 Protect the Gut During Candida albicans Infections Enhancing the Intestinal Barrier Integrity: In Vitro Study. Front. Cell. Infect. Microbiol. 2021, 11, 666900. [Google Scholar] [CrossRef]
- Fusco, A.; Savio, V.; Cammarota, M.; Alfano, A.; Schiraldi, C.; Donnarumma, G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017, 2017, 6976935. [Google Scholar] [CrossRef] [Green Version]
- Fusco, A.; Savio, V.; Stelitano, D.; Baroni, A.; Donnarumma, G. The Intestinal Biofilm of Pseudomonas aeruginosa and Staphylococcus aureus Is Inhibited by Antimicrobial Peptides HBD-2 and HBD-3. Appl. Sci. 2021, 11, 6595. [Google Scholar] [CrossRef]












| Name | Sample Description 1 |
|---|---|
| CN | Chitin nanofibrils |
| CN-PEG | CN with PEG8000 (2 wt%) |
| CN-NL | CN-NL complex with PEG8000 (2 wt%) |
| CN-NL/GA | CN-NL + glycyrrhetinic acid (0.2 wt%) |
| CN-NL/VE | CN-NL + Vitamin E (0.2 wt%) |
| CN-NL/VC | CN-NL + Sodium ascorbyl phosphate (0.2 wt%) |
| CN-NL/LU | CN-NL + lutein (0.5 wt%) |
| CN-NL/NI | CN-NL + nicotinamide (5 wt%) |
| CN-NL/AG | CN-NL + silver nanoparticles (0.1 wt%) |
| CN/AG | CN + silver nanoparticles (0.1 wt%) |
| Gene | Primers Sequence | Conditions | Product Size (bp) |
|---|---|---|---|
| IL-1 α | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | 5″ at 95 °C, 8″ at 55 °C, 17″ at 72 °C for 45 cycles | 421 |
| TNF-α | 5′-CAGAGGGAAGAGTTCCCCAG-3′ 5′-CCTTGGTCTGGTAGGAGACG-3′ | 5″ at 95 °C, 6″ at 57 °C, 13″ at 72 °C for 40 cycles | 324 |
| IL-6 | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | 5″ at 95 °C, 13″ at 56 °C, 25″ at 72 °C for 40 cycles | 628 |
| IL-8 | 5-ATGACTTCCAAGCTGGCCGTG-3′ 5-TGAATTCTCAGCCCTCTTCAAAAACTTCTC | 5′’ at 94 °C, 6″ at 55 °C, 12″ at 72 °C for 40 cycles | 297 |
| TGF-β | 5′-CCGACTACTACGCCAAGGAGGTCAC-3′ 5′-AGGCCGGTTCATGCCATGAATGGTG-3′ | 5″ at 94 °C, 9″ at 60 °C, 18″ at 72 °C for 40 cycles | 439 |
| IL-1 β | 5′-GCATCCAGCTACGAATCTCC-3′ 5′-CCACATTCAGCACAGGACTC-3′ | 5″ at 95 °C, 14″ at 58 °C, 28″ at 72 °C for 40 cycles | 708 |
| Pure CN | NL | PEG | CN (PEG) | CN-NL (PEG) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tinfl (°C) | Δwt% | Tinfl (°C) | Δwt% | Tinfl (°C) | Δwt% | Tinfl (°C) | Δwt% | Tinfl (°C) | Δwt% |
| 51.7 191.2 349.1 394.2 514.4 | −2.4 −2.6 −21.2 −9.5 −2.7 | 72.2 361.149 | −5.26 −55.63 | 406.2 | −97.0 | 46.5 188.6 329.8 421.6 471.3 | −1.9 −5.3 −57.5 −17.8 | 49.7 130.5 292.5 430 | −5.3 −2.6 −69.2 −10.4 |
| R900 = 58.1 wt% | R900 = 38.9 wt% | R900 = 2.8 wt% | R900 = 17.4 wt% | R900 = 12.4 wt% | |||||
| 5%ML: 140 °C | 5%ML: 140 °C | 5%ML: 320 °C | 5%ML: 140 °C | 5%ML: 100 °C | |||||
| Samples | 5%ML (°C) | Inflection Point (Main Loss) (°C) (wt%) | Step (Main Loss) (%) | Residue (wt%) |
|---|---|---|---|---|
| CN/AG | 240 | 413.6 | 86.4 | 12.01 |
| GA | 240 | 404.1 | 98.9 | 1.97 |
| VE | 180 | 367.4 | 99.8 | 0.4 |
| VC | 70 | 279.1 | 41.5 | 58.5 |
| NI | 140 | 266.7 | 99.7 | 0.3 |
| LU | 180 | 416.3 | 98.6 | 1.4 |
| Samples (a) | m (wt%) | 5%ML | Onset 1 (°C) | Step 1 (wt%) | Onset 2 (°C) | Step 2 (wt%) | Onset 3 (°C) | Step 3 (wt%) | Residue (wt%) |
|---|---|---|---|---|---|---|---|---|---|
| CN (PEG) | - | 140 | 159 | −5.35 | 300 | −57.54 | 387 | −17.85 | 17.42 |
| CN-NL (wt ratio 2:1) | - | 100 | 114 | −2.599 | 266 | −69.25 | 406 | −10.39 | 12.37 |
| CN-NL/LU | 0.5 | 110 | 123 | −1.637 | 253 | −73.74 | 387 | −8.197 | 11.78 |
| CN-NL/NI | 5 | 140 | 189 | −23.6 | 276 | −48.58 | 438 | −13.99 | 11.80 |
| CN-NL/VC | 0.5 | 50 | 80 | −10 | 267 | −56.45 | 373 | −14.02 | 18.91 |
| CN-NL/AG | 0.2 | 200 | 217 | −14.88 | 292 | −68.99 | 416 | −10.37 | 4.41 |
| CN-NL/GA | 0.2 | 100 | 127 | −1.92 | 251 | −68.53 | 472 | −11.63 | 13.84 |
| CN-NL/VE | 0.2 | 100 | 125 | −2.545 | 264 | −70.99 | 435 | −10.90 | 11.56 |
| CN-LIG + AgNPs | CN-LIG + Vit.E 0.2% | CN-LIG + Sodium Ascorbyl phosphate | CN-LIG+ Lutein 0.5% | CN-LIG | CN-LIG + Niacinamide 5% | CN | CN-LIG + Glycyrrhetinic Acid 0.2% | |
|---|---|---|---|---|---|---|---|---|
| 1:10 | 0.05 | 0.1 | 0.55 | 0.16 | 0.67 | 0.4 | 1.45 * | 0.12 |
| 1:20 | 0.0475 | 0.096 | 0.67 | 0.2 | 0.7 | 0.5 | 1.4 | 0.33 |
| 1:50 | 0.044 | 0.115 | 0.66 | 0.56 | 0.77 | 0.48 | 1.4 | 1.02 |
| 1:100 | 0.048 | 0.5 | 0.74 | 0.55 | 0.7 | 0.5 | 1.2 | 1.05 |
| 1:200 | 0.046 | 0.52 | 0.68 | 0.68 | 0.75 | 0.6 | 1.25 | 1.25 * |
| 1:500 | 0.145 | 0.98 | 1.2 * | 0.99 | 1.34 * | 0.7 | 0.95 | 0.99 |
| 1:1000 | 0.173 | 0.98 | 1.04 | 0.92 | 1.1 ± 5 | 0.74 | 0.9 | 0.95 |
| 1:1500 | 0.7 | 0.92 | 0.93 | 0.98 | 0.96 | 0.98 * | 0.92 | 0.93 |
| 1:2000 | 0.77 * | 1.13 * | 0.87 | 1.12 * | 0.99 | 0.88 | 0.87 | 0.95 |
| 1:4000 | 0.756 | 0.93 | 0.9 | 0.9 | 0.98 | 0.92 | 0.93 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coltelli, M.-B.; Morganti, P.; Castelvetro, V.; Lazzeri, A.; Danti, S.; Benjelloun-Mlayah, B.; Gagliardini, A.; Fusco, A.; Donnarumma, G. Chitin Nanofibril-Nanolignin Complexes as Carriers of Functional Molecules for Skin Contact Applications. Nanomaterials 2022, 12, 1295. https://doi.org/10.3390/nano12081295
Coltelli M-B, Morganti P, Castelvetro V, Lazzeri A, Danti S, Benjelloun-Mlayah B, Gagliardini A, Fusco A, Donnarumma G. Chitin Nanofibril-Nanolignin Complexes as Carriers of Functional Molecules for Skin Contact Applications. Nanomaterials. 2022; 12(8):1295. https://doi.org/10.3390/nano12081295
Chicago/Turabian StyleColtelli, Maria-Beatrice, Pierfrancesco Morganti, Valter Castelvetro, Andrea Lazzeri, Serena Danti, Bouchra Benjelloun-Mlayah, Alessandro Gagliardini, Alessandra Fusco, and Giovanna Donnarumma. 2022. "Chitin Nanofibril-Nanolignin Complexes as Carriers of Functional Molecules for Skin Contact Applications" Nanomaterials 12, no. 8: 1295. https://doi.org/10.3390/nano12081295