Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers
Abstract
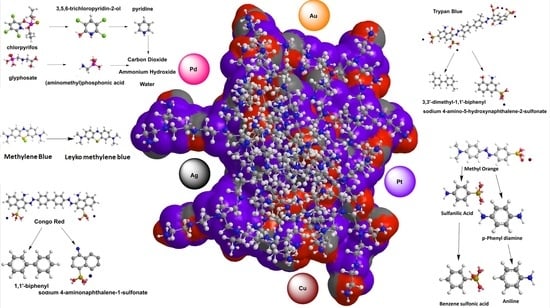
:1. Introduction
2. Conventional Homogenous Catalysis by Metal Nanoparticles
2.1. Reduction of Aromatic Nitro-Compounds
2.1.1. The Benchmark Reaction of p-Nitrophenol


2.1.2. Aromatic Nitro-Derivates in General

2.2. Treatment of Pigments
3. Formulations and Solid Supports for Heterogeneous Catalysis
3.1. Early Evolution Efforts by Following the p-Nitrophenol Standard



3.2. Heterogeneous Catalytic Degradation of Dyes
3.3. Applications of Heterogenous Catalysis in the Treatment of Other Water Contaminants
4. Unconventional Catalysis Involving Dendritic Polymers


5. Photocatalytic Decomposition
5.1. Photocatalysis on the Prominent TiO2 Substrate

5.2. Photocatalysis with Other Photoactive NPs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.; Sun, W.; Shi, X. Design and Biomedical Applications of Poly(amidoamine)-Dendrimer-Based Hybrid Nanoarchitectures. Small Methods 2017, 1, 1700224. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Petrakli, F.; Arkas, M.; Tsetsekou, A. α-Alumina nanospheres from nano-dispersed boehmite synthesized by a wet chemical route. J. Am. Ceram. Soc. 2018, 101, 3508–3519. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, M.; Sharma, N.K.; Sajal, V. Localized surface plasmon resonance based fiber optic sensor with nanoparticles. Opt. Commun. 2013, 292, 92–100. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. A Review of Dendrimer-Encapsulated Metal Nanocatalysts Applied in the Fine Chemical Transformations. Catal. Lett. 2018, 149, 84–99. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Shah, M.P. Bioremediation-Waste Water Treatment. J. Bioremediat. Biodegrad. 2018, 9, 427. [Google Scholar] [CrossRef]
- Golka, K.; Kopps, S.; Myslak, Z.W. Carcinogenicity of azo colorants: Influence of solubility and bioavailability. Toxicol. Lett. 2004, 151, 203–210. [Google Scholar] [CrossRef]
- Akarslan, F.; Demiralay, H. Effects of textile materials harmful to human health. Acta Phys. Polonica A 2015, 128, 407–409. [Google Scholar] [CrossRef]
- Brüschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Said, S.; Mikhail, S.; Riad, M. Recent progress in preparations and applications of meso-porous alumina. Mater. Sci. Energy Technol. 2019, 2, 288–297. [Google Scholar] [CrossRef]
- Vadivel, S.; Naveen, A.; Theerthagiri, J.; Madhavan, J.; Priya, T.S.; Balasubramanian, N. Solvothermal synthesis of BiPO4 nanorods/MWCNT (1D-1D) composite for photocatalyst and supercapacitor applications. Ceram. Int. 2016, 42, 14196–14205. [Google Scholar] [CrossRef]
- Arkas, M.; Panagiotaki, K.; Kitsou, I.; Petrakli, F. Dendritic Polymer—Enhanced Ultrafiltration. In Micro and Nano Technologies 2018, Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–152. [Google Scholar] [CrossRef]
- Strickland, A.F.; Perkins, W.S. Decolorization of continuous dyeing wastewater by ozonation. Text. Chem. Colorist 1995, 27, 11–15. [Google Scholar]
- Kornaros, M.; Lyberatos, G. Biological treatment of wastewaters from a dye manufacturing company using a trickling filter. J. Hazard. Mater. 2006, 136, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-L.; Wang, B.; Zeng, G.-M.; Yang, C.; Niu, C.-G.; Niu, Q.-Y.; Zhou, W.-J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Tully, D.C.; Fréchet, J.M.J. Dendrimers at surfaces and interfaces: Chemistry and applications. Chem. Commun. 2001, 14, 1229–1239. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M.-A. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Kim, Y.H. Hyperbranched polymers 10 years after. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1685–1698. [Google Scholar] [CrossRef]
- Sunder, A.; Heinemann, J.; Frey, H. Controlling the growth of polymer trees: Concepts and perspectives for hyperbranched polymers. Chem. A Eur. J. 2000, 6, 2499–2506. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Arkas, M. Columnar and smectic self-assembly deriving from non ionic amphiphilic hyperbranched polyethylene imine polymers and induced by hydrogen bonding and segregation into polar and non polar parts. Polymer 2013, 54, 1114–1122. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Jean DA, T.; Fréchet, M.J.; Tomalia, D.A. Dendrimers and Other Dendritic Polymers; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Grayson, S.M.; Frechet, J. Convergent Dendrons and Dendrimers: From Synthesis to Applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Schlüter, A.D.; Rabe, J.P. Dendronized polymers: Synthesis, characterization, assembly at interfaces, and manipulation. Angew. Chem. Int. Ed. 2000, 39, 864–883. [Google Scholar] [CrossRef]
- Yates, C.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional Dendrimeric “Nanosponges” for the Removal of Polycyclic Aromatic Hydrocarbons from Water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Aliannejadi, S.; Hassani, A.H.; Panahi, H.A.; Borghei, S.M. Preparation and characterization of a recyclable high-branched/generation dendrimer nano-polymer based on enhanced magnetic core for naphthalene sorption from aqueous solutions. Desalin. Water Treat. 2020, 202, 364–380. [Google Scholar] [CrossRef]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Res. 2007, 41, 476–486. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon Dendritic Networks in Porous Ceramics for Water Purification. Chem. Mater. 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Newkome, G.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef] [Green Version]
- Newkome, G.R.; Moorefield, C.N.; Baker, G.R.; Johnson, A.L.; Behera, R.K. Alkane Cascade Polymers Possessing Micellar Topology: Micellanoic Acid Derivatives. Angew. Chem. Int. Ed. 1991, 30, 1176–1178. [Google Scholar] [CrossRef]
- Kim, Y.H.; Webster, O.W. Water soluble hyperbranched polyphenylene: “A Unimolecular Micelle?”. J. Am. Chem. Soc. 1990, 112, 4592–4593. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Baker, G.R.; Saunders, M.J.; Grossman, S.H. Unimolecular Micelles. Angew. Chem. Int. Ed. 1991, 30, 1178–1180. [Google Scholar] [CrossRef]
- Hawker, C.J.; Wooley, K.L.; Fréchet, J.M.J. Unimolecular micelles and globular amphiphiles: Dendritic macromolecules as novel recyclable solubilization agents. J. Chem. Soc. Perkin Trans. 1 1993, 12, 1287–1297. [Google Scholar] [CrossRef]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168–2203. [Google Scholar] [CrossRef]
- Arkas, M.; Kitsou, I.; Gkouma, A.; Papageorgiou, M. The role of hydrogen bonds in the mesomorphic behaviour of supramolecular assemblies organized in dendritic architectures. Liq. Cryst. Rev. 2019, 7, 60–105. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2000, 34, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional Dendritic Polymers for the Development of Hybrid Materials for Water Purification. Macromol. Mater. Eng. 2010, 295, 883–898. [Google Scholar] [CrossRef]
- Arkas, M. Hybrid organoceramics deriving from dendritic polymers, methods of preparation, optimization techniques and prospected applications. In Recent Advances in Ceramic Materials Research (Materials Science and Technologies); Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly(ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Xu, X.; Lü, S.; Gao, C.; Bai, X.; Feng, C.; Gao, N.; Liu, M. Multifunctional drug carriers comprised of mesoporous silica nanoparticles and polyamidoamine dendrimers based on layer-by-layer assembly. Mater. Des. 2015, 88, 1127–1133. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Xing, D.; Shao, W.-J.; Du, X.-Y.; La, P.-Q. Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg(II) in aqueous solution. J. Colloid Interface Sci. 2017, 505, 352–363. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Lotfi, R. Functional dendritic compounds: Potential prospective candidates for dental restorative materials and in situ re-mineralization of human tooth enamel. RSC Adv. 2016, 6, 43127–43146. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Tsetsekou, A.; Kammenou, M.-I.; Boukos, N. Controlling the Formation of Hydroxyapatite Nanorods with Dendrimers. J. Am. Ceram. Soc. 2011, 94, 2023–2029. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control. Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Leiro, V.; Moreno, P.M.; Sarmento, B.; Durão, J.; Gales, L.; Pêgo, A.P.; Barrias, C.C. Design and preparation of biomimetic and bioinspired materials. In Bioinspired Materials for Medical Applications; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 1–44. [Google Scholar] [CrossRef]
- Acosta, E.J.; Gonzalez, S.O.; Simanek, E.E. Synthesis, characterization, and application of melamine-based dendrimers supported on silica gel. J. Polym. Sci. Part A Polym. Chem. 2004, 43, 168–177. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Vardavoulias, M.; Arkas, M. Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications. Nanomaterials 2020, 11, 19. [Google Scholar] [CrossRef]
- Mourey, T.H.; Turner, S.R.; Rubinstein, M.; Frechet, J.; Hawker, C.J.; Wooley, K.L. Unique behavior of dendritic macromolecules: Intrinsic viscosity of polyether dendrimers. Macromolecules 1992, 25, 2401–2406. [Google Scholar] [CrossRef]
- Arkas, M.; Allabashi, R.; Tsiourvas, D.; Mattausch, E.-M.; Perfler, R. Organic/Inorganic Hybrid Filters Based on Dendritic and Cyclodextrin “Nanosponges” for the Removal of Organic Pollutants from Water. Environ. Sci. Technol. 2006, 40, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Eleades, L.; Paleos, C.M.; Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 2005, 97, 2299–2305. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Arkas, M.; Kritikaki, A.; Simonetis, S.; Tsiourvas, D. Optimization of hybrid hyperbranched polymer/ceramic filters for the efficient absorption of polyaromatic hydrocarbons from water. J. Membr. Sci. 2007, 311, 128–135. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Nanometals: Formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Kurczewska, J.; Schroeder, G. Nanomaterials Modification by Dendrimers—A Review. World J. Res. Rev. 2018, 6, 262658. [Google Scholar]
- Sadjadi, S.; Sadjadi, S. Dendritic polymers for environmental remediation. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–335. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Ali, F.; Al-Harthi, M.A. Dendrimer assisted dye-removal: A critical review of adsorption and catalytic degradation for wastewater treatment. J. Mol. Liq. 2020, 315, 113775. [Google Scholar] [CrossRef]
- Zhao, M.; Crooks, R.M. Homogeneous hydrogenation catalysis with monodisperse, dendrimer-encapsulated Pd and Pt nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu Nanoclusters within Dendrimer Templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usui, A.K.; Torigoe, K. Preparation of Gold Colloids with UV Irradiation Using Dendrimers as Stabilizer. Langmuir 1998, 14, 3157–3159. [Google Scholar] [CrossRef]
- Elia, M.C.; Storer, R.D.; McKelvey, T.W.; Kraynak, A.R.; Barnum, J.E.; Harmon, L.S.; Deluca, J.G.; Nichols, W.W. Rapid DNA degradation in primary rat hepatocytes treated with diverse cytotoxic chemicals: Analysis by pulsed field gel electrophoresis and implications for alkaline elution assays. Environ. Mol. Mutagen. 1994, 24, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, L.A.; Logsdon, T.R.; Copeland, F.; Beyer, P.E.; Kavlock, R.J.; Ebron-McCoy, M.T. In vitro embryotoxicity of a series of para-substituted phenols: Structure, activity, and correlation with in vivo data. Teratology 1992, 45, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Yoshimura, T.; Esumi, K. Preparation of Gold−Dendrimer Nanocomposites by Laser Irradiation and Their Catalytic Reduction of 4-Nitrophenol. Langmuir 2003, 19, 5517–5521. [Google Scholar] [CrossRef]
- Esumi, K.; Isono, A.R.; Yoshimura, T. Preparation of PAMAM-and PPI-Metal (Silver, Platinum, and Palladium) Nanocomposites and Their Catalytic Activities for Reduction of 4-Nitrophenol. Langmuir 2003, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bingwa, N.; Meijboom, R. Evaluation of catalytic activity of Ag and Au dendrimer-encapsulated nanoparticles in the reduction of 4-nitrophenol. J. Mol. Catal. A Chem. 2015, 396, 1–7. [Google Scholar] [CrossRef]
- Feng, Z.V.; Lyon, J.L.; Croley, J.S.; Crooks, R.M.; Bout, D.A.V.; Stevenson, K. Synthesis and Catalytic Evaluation of Dendrimer-Encapsulated Cu Nanoparticles. An Undergraduate Experiment Exploring Catalytic Nanomaterials. J. Chem. Educ. 2009, 86, 368. [Google Scholar] [CrossRef]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-Dependent Hydrogenation of p-Nitrophenol with Pd Nanoparticles Synthesized with Poly(amido)amine Dendrimer Templates. J. Phys. Chem. C 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J. Colloid Interface Sci. 2013, 389, 260–267. [Google Scholar] [CrossRef]
- Noh, J.H.; Meijboom, R. Catalytic evaluation of dendrimer-templated Pd nanoparticles in the reduction of 4-nitrophenol using Langmuir–Hinshelwood kinetics. Appl. Surf. Sci. 2014, 320, 400–413. [Google Scholar] [CrossRef]
- Bingwa, N.; Meijboom, R. Kinetic Evaluation of Dendrimer-Encapsulated Palladium Nanoparticles in the 4-Nitrophenol Reduction Reaction. J. Phys. Chem. C 2014, 118, 19849–19858. [Google Scholar] [CrossRef]
- Patala, R.; Noh, J.-H.; Meijboom, R. Determination of maximum loading capacity of polyamidoamine (PAMAM) dendrimers and evaluation of Cu55 dendrimer-encapsulated nanoparticles for catalytic activity. Int. J. Chem. Kinet. 2018, 50, 693–704. [Google Scholar] [CrossRef]
- Noh, J.H.; Meijboom, R. Synthesis and catalytic evaluation of dendrimer-templated and reverse microemulsion Pd and Pt nanoparticles in the reduction of 4-nitrophenol: The effect of size and synthetic methodologies. Appl. Catal. A Gen. 2015, 497, 107–120. [Google Scholar] [CrossRef]
- Endo, T.; Yoshimura, T.; Esumi, K. Synthesis and catalytic activity of gold-silver binary nanoparticles stabilized by PAMAM dendrimer. J. Colloid Interface Sci. 2005, 286, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Croley, J.S.; Stevenson, K.J. Electroreduction of p-Nitrophenol at Supported Mono-and Bimetallic Dendrimer Encapsulated Catalysts. UT-Austin 2007–2008 Beckman Scholars Final Report. pp. 1–21. Available online: https://www.preprints.org/manuscript/202112.0451/v1 (accessed on 20 December 2021).
- Antonels, N.C.; Meijboom, R. Preparation of well-defined dendrimer Encapsulated ruthenium nanoparticles and their evaluation in the reduction of 4-nitrophenol according to the Langmuir-Hinshelwood approach. Langmuir 2013, 29, 13433–13442. [Google Scholar] [CrossRef] [PubMed]
- Marvin, K.A.; Thadani, N.N.; Atkinson, C.A.; Keller, E.L.; Stevenson, K.J. Preparation and catalytic evaluation of ruthenium–nickel dendrimer encapsulated nanoparticles via intradendrimer redox displacement of nickel nanoparticles. Chem. Commun. 2012, 48, 6289–6291. [Google Scholar] [CrossRef] [Green Version]
- Gatard, S.; Salmon, L.; Deraedt, C.; Ruiz, J.; Astruc, D.; Bouquillon, S. Gold Nanoparticles Stabilized by Glycodendrimers: Synthesis and Application to the Catalytic Reduction of 4-Nitrophenol. Eur. J. Inorg. Chem. 2014, 2014, 2671–2677. [Google Scholar] [CrossRef]
- Gatard, S.; Salmon, L.; Deraedt, C.; Ruiz, J.; Astruc, D.; Bouquillon, S. Palladium Nanoparticles Stabilized by Glycodendrimers and Their Application in Catalysis. Eur. J. Inorg. Chem. 2014, 2014, 4369–4375. [Google Scholar] [CrossRef]
- Li, N.; Echeverría, M.; Moya, S.; Ruiz, J.; Astruc, D. “Click” Synthesis of Nona-PEG-branched Triazole Dendrimers and Stabilization of Gold Nanoparticles That Efficiently Catalyze p-Nitrophenol Reduction. Inorg. Chem. 2014, 53, 6954–6961. [Google Scholar] [CrossRef]
- Liu, X.; Ruiz, J.; Astruc, D. Compared Catalytic Efficiency of Click-Dendrimer-Stabilized Late Transition Metal Nanoparticles in 4-Nitrophenol Reduction. J. Inorg. Organomet. Polym. Mater. 2018, 28, 399–406. [Google Scholar] [CrossRef]
- Tanaka, H.; Hashimoto, T.; Koizumi, S.; Itoh, H.; Naka, K.; Chujo, Y. Control of Self-Assembling Processes of Polyamidoamine Dendrimers and Pd Nanoparticles. Macromolecules 2008, 41, 1815–1824. [Google Scholar] [CrossRef]
- Kannan, A.; Rajakumar, P. Synthesis and catalytic application of glycodendrimers decorated with gold nanoparticles – reduction of 4-nitrophenol. RSC Adv. 2015, 5, 46908–46915. [Google Scholar] [CrossRef]
- Patil, N.G.; Basutkar, N.B.; Ambade, A.V. Copper and silver nanoparticles stabilized by bistriazole-based dendritic amphiphile micelles for 4-nitrophenol reduction. New J. Chem. 2017, 41, 4546–4554. [Google Scholar] [CrossRef]
- Liu, X.; Mu, S.; Long, Y.; Qiu, G.; Ling, Q.; Gu, H.; Lin, W. Gold Nanoparticles Stabilized by 1,2,3-Triazolyl Dendronized Polymers as Highly Efficient Nanoreactors for the Reduction of 4-Nitrophenol. Catal. Lett. 2019, 149, 544–551. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Wang, Y.; Gu, H. Ferrocene-containing amphiphilic dendronized random copolymer as efficient stabilizer for reusable gold nanoparticles in catalysis. React. Funct. Polym. 2019, 143, 104325. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Astruc, D.; Lin, W.; Gu, H. Highly-branched amphiphilic organometallic dendronized diblock copolymer: ROMP synthesis, self-assembly and long-term Au and Ag nanoparticle stabilizer for high-efficiency catalysis. Polymer 2019, 173, 1–10. [Google Scholar] [CrossRef]
- Asharani, I.V.; Thirumalai, D. Synthesis of Dendrimer-Encapsulated Silver Nanoparticles and Its Catalytic Activity on the Reduction of 4-Nitrophenol. J. Chin. Chem. Soc. 2012, 59, 1455–1460. [Google Scholar] [CrossRef]
- Gürbüz, M.U.; Ertürk, A.S. Synthesis and Characterization of Jeffamine Core PAMAM Dendrimer-Silver Nanocomposites (Ag JCPDNCs) and Their Evaluation in The Reduction of 4-Nitrophenol. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 885–894. [Google Scholar] [CrossRef]
- Murugan, E.; Pakrudheen, I. Efficient Amphiphilic Poly(propylene imine) Dendrimer Encapsulated Ruthenium Nanoparticles for Sensing and Catalysis Applications. Sci. Adv. Mater. 2015, 7, 891–901. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.; Yuan, Y.; Chen, Y.; Cheng, F.; Jiang, S.-C. Amphiphilic hyperbranched copolymers bearing a hyperbranched core and a dendritic shell as novel stabilizers rendering gold nanoparticles with an unprecedentedly long lifetime in the catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 21173–21182. [Google Scholar] [CrossRef]
- Gao, L.; Nishikata, T.; Kojima, K.; Chikama, K.; Nagashima, H. Water-and Organo-Dispersible Gold Nanoparticles Supported by Using Ammonium Salts of Hyperbranched Polystyrene: Preparation and Catalysis. Chem. Asian J. 2013, 8, 3152–3163. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, X.; Cao, M. Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol. Catalysts 2015, 6, 3. [Google Scholar] [CrossRef]
- Redón, R.; Ramírez-Crescencio, F.; Gonzalez-Rodriguez, R.; Coffer, J.; Simanek, E.E. Ir(0) and Pt(0) nanoparticle-triazine dendrimer composites. J. Coord. Chem. 2020, 73, 544–557. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Cui, Y.; Gao, D.; Kang, J.; Sun, H.; Zhu, L.; Chen, S. Highly stable and biocompatible dendrimer-encapsulated gold nanoparticle catalysts for the reduction of 4-nitrophenol. New J. Chem. 2017, 41, 8399–8406. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, R.; Zhao, T.; Sun, T.; Chu, X.; Liu, S.; Tang, E.; Xu, X. Efficient reduction of 4-nitrophenol catalyzed by 4-carbo-methoxypyrrolidone modified PAMAM dendrimer–silver nanocomposites. Catal. Sci. Technol. 2019, 9, 6145–6151. [Google Scholar] [CrossRef]
- Lim, J.; Mintzer, M.A.; Perez, L.; Simanek, E.E. Synthesis of Odd Generation Triazine Dendrimers Using a Divergent, Macromonomer Approach. Org. Lett. 2010, 12, 1148–1151. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Yu, P.; Zhang, X.; Zhuo, R. Gold nanoparticles stabilized by amphiphilic hyperbranched polymers for catalytic reduction of 4-nitrophenol. J. Catal. 2016, 337, 65–71. [Google Scholar] [CrossRef]
- Dong, H.; Dai, Y.; Zhang, X.; Zhang, Z.; Fu, S.; Zhong, Z. The influence of amine structures on the stability and catalytic activity of gold nanoparticles stabilized by amine-modified hyperbranched polymers. Nanotechnology 2017, 29, 055705. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Zhuo, R. Amphiphilic linear-hyperbranched polymer poly(ethylene glycol)-branched polyethylenimine-poly(ϵ-caprolactone): Synthesis, self-assembly and application as stabilizer of platinum nanoparticles. Polym. Int. 2016, 65, 691–697. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, W.; Du, Y.; Wang, X. Hydrogenation of nitrobenzenes catalyzed by platinum nanoparticle core-polyaryl ether trisacetic acid ammonium chloride dendrimer shell nanocomposite. J. Mol. Catal. A Chem. 2006, 260, 4–10. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Du, Y.; Wang, X.; Yang, P. Gold/Platinum Bimetallic Core/Shell Nanoparticles Stabilized by a Fréchet-Type Dendrimer: Preparation and Catalytic Hydrogenations of Phenylaldehydes and Nitrobenzenes. Catal. Lett. 2008, 127, 429–436. [Google Scholar] [CrossRef]
- Murugan, E.; Rangasamy, R.; Pakrudheen, I. Efficient Amphiphilic Poly(propyleneimine) Dendrimer Stabilized Gold Nanoparticle Catalysts for Aqueous Phase Reduction of Nitrobenzene. Sci. Adv. Mater. 2012, 4, 1103–1110. [Google Scholar] [CrossRef]
- Asharani, I.V.; Thirumalai, D.; Sivakumar, A. Dendrimer encapsulated silver nanoparticles as novel catalysts for reduction of aromatic nitro compounds. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 263, p. 022010. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, V.A.; Marrugo, K.; Campos, C.H.; Alderete, J.B.; Torres, C.C. Copper metallic nanoparticles capped with PEGylated PAMAM-G3 dendrimers for the catalytic reduction of low solubility nitroarenes of pharmaceutical interest. Catal. Today 2020, 372, 27–35. [Google Scholar] [CrossRef]
- Campos, C.H.; Bustamante, T.M.; Jiménez, V.A.; Torres, C.C.; Alderete, J.B. Efficient and recyclable gold nanoparticles as catalysts for the cleaner production of 4-morpholinoanilines used as pharmaceutical building blocks. J. Clean. Prod. 2020, 290, 125761. [Google Scholar] [CrossRef]
- Sanyal, M.; Sharma, U. PAMAM (poly-amido amine) dendrimer supported copper nanoparticles for chemoselective nitro reduction. J. Indian Chem. Soc. 2021, 98, 100149. [Google Scholar] [CrossRef]
- Murugan, E.; Pakrudheen, I.; Gomathi, G. Amphiphilic dendrimer stabilized Ag, Pd and Pt homogeneous nanoparticle catalysts and their catalysis for the reduction of methyl orange. In Proceedings of the International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, India, 28–30 November 2011; IEEE: New York, NY, USA, 2011; pp. 35–39. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Khoza, T.; Tjabadi, E.; Meijboom, R. Effective Catalytic Reduction of Methyl Orange Catalyzed by the Encapsulated Random Alloy Palladium-Gold Nanoparticles Dendrimer. ChemistrySelect 2017, 2, 9803–9809. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Synthesis of narrowly dispersed silver and gold nanoparticles and their catalytic evaluation for morin oxidation. Appl. Catal. A Gen. 2016, 509, 17–29. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. Catalytic Behavior of Different Sizes of Dendrimer-Encapsulated Aun Nanoparticles in the Oxidative Degradation of Morin with H2O2. Langmuir 2015, 31, 9041–9053. [Google Scholar] [CrossRef]
- Ncube, P.; Hlabathe, T.; Meijboom, R. The preparation of well-defined dendrimer-encapsulated palladium and platinum nanoparticles and their catalytic evaluation in the oxidation of morin. Appl. Surf. Sci. 2015, 357, 1141–1149. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Catalytic and kinetic investigation of the encapsulated random alloy (Pdn-Au110-n) nanoparticles. Appl. Catal. B Environ. 2016, 189, 86–98. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, R.; Dong, L.; Cui, Y.; Chen, S.; Sun, H.; Ma, G.; Gao, D.; Wang, L. Biocompatible Dendrimer-Encapsulated Palladium Nanoparticles for Oxidation of Morin. ACS Omega 2019, 4, 18685–18691. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, X.; Chen, S.; Cui, Y.; Yu, Q.; Yang, L.; Sun, H.; Gao, D.; Xie, D. Highly stable and biocompatible zwitterionic dendrimer-encapsulated palladium nanoparticles that maintain their catalytic activity in bacterial solution. New J. Chem. 2018, 42, 19740–19748. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Catalytic oxidation of methylene blue by dendrimer encapsulated silver and gold nanoparticles. J. Mol. Catal. A Chem. 2016, 411, 48–60. [Google Scholar] [CrossRef]
- Ncube, P.; Bingwa, N.; Baloyi, H.; Meijboom, R. Catalytic activity of palladium and gold dendrimer-encapsulated nanoparticles for methylene blue reduction: A kinetic analysis. Appl. Catal. A Gen. 2015, 495, 63–71. [Google Scholar] [CrossRef]
- Murugan, E.; Rangasamy, R. Synthesis, characterization, and heterogeneous catalysis of polymer-supported poly(propyleneimine) dendrimer stabilized gold nanoparticle catalyst. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2525–2532. [Google Scholar] [CrossRef]
- Dang, G.; Shi, Y.; Fu, Z.; Yang, W. Polymer particles with dendrimer@SiO2–Ag hierarchical shell and their application in catalytic column. J. Colloid Interface Sci. 2011, 369, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, W.; Quinlan, P.J.; Berry, R.M.; Tam, M.K. Sustainable Catalysts from Gold-Loaded Polyamidoamine Dendrimer-Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Wang, M.-L.; Jiang, T.-T.; Lu, Y.; Liu, H.-J.; Chen, Y. Gold nanoparticles immobilized in hyperbranched polyethylenimine modified polyacrylonitrile fiber as highly efficient and recyclable heterogeneous catalysts for the reduction of 4-nitrophenol. J. Mater. Chem. A 2013, 1, 5923–5933. [Google Scholar] [CrossRef]
- Hu, D.; Huang, Y.; Liu, H.; Wang, H.; Wang, S.; Shen, M.; Zhu, M.; Shi, X. The assembly of dendrimer-stabilized gold nanoparticles onto electrospun polymer nanofibers for catalytic applications. J. Mater. Chem. A 2013, 2, 2323–2332. [Google Scholar] [CrossRef]
- Li, H.; Lü, J.; Zheng, Z.; Cao, R. An efficient and reusable silica/dendrimer supported platinum catalyst for electron transfer reactions. J. Colloid Interface Sci. 2011, 353, 149–155. [Google Scholar] [CrossRef]
- Ricciardi, R.; Huskens, J.; Verboom, W. Influence of the Au/Ag ratio on the catalytic activity of dendrimer-encapsulated bimetallic nanoparticles in microreactors. J. Flow Chem. 2015, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Gaofei, D.A.N.G.; Yan, S.H.I.; Zhifeng, F.U.; Wantai, Y.A.N.G. Fe3O4@PS@PAMAM-Ag magnetic nanocatalysts and their recoverable catalytic ability. Chin. J. Catal. 2012, 33, 651–658. [Google Scholar] [CrossRef]
- Kurtan, U.; Baykal, A. Fabrication and characterization of Fe3O4 @APTES@PAMAM-Ag highly active and recyclable magnetic nanocatalyst: Catalytic reduction of 4-nitrophenol. Mater. Res. Bull. 2014, 60, 79–87. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Li, W.; Feng, R.; Li, Z.; Lyu, P.; Ma, Y. Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2018, 54, 323–334. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Cui, G.; Tian, Y.; Yan, S. Multifunctional magnetic core–shell dendritic mesoporous silica nanospheres decorated with tiny Ag nanoparticles as a highly active heterogeneous catalyst. Appl. Surf. Sci. 2016, 360, 252–262. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; AlZahrani, I.I.; Thawibaraka, H.I.; Naglah, A.M.; El-Desouky, M.G.; El-Bindary, M.A. Adsorption studies of carbon dioxide and anionic dye on green adsorbent. J. Mol. Struct. 2021, 1250, 131736. [Google Scholar] [CrossRef]
- Rajesh, R.; Venkatesan, R. Encapsulation of silver nanoparticles into graphite grafted with hyperbranched poly(amidoamine) dendrimer and their catalytic activity towards reduction of nitro aromatics. J. Mol. Catal. A Chem. 2012, 359, 88–96. [Google Scholar] [CrossRef]
- Ramezanpour, A.; Karami, K.; Kharaziha, M.; Silvestru, C.; Bayat, P. Synthesis and characterization of the ternary graphene oxide, MnFe2O4 nanoparticles, and Polyamidoamine dendrons nanocomposite decorated with palladium as a heterogeneous catalyst for nitroaromatics reduction. Appl. Organomet. Chem. 2021, 35, e6329. [Google Scholar] [CrossRef]
- Murugan, E.; Vimala, G. Synthesis, characterization, and catalytic activity for hybrids of multi-walled carbon nanotube and amphiphilic poly(propyleneimine) dendrimer immobilized with silver and palladium nanoparticle. J. Colloid Interface Sci. 2013, 396, 101–111. [Google Scholar] [CrossRef]
- Paleos, C.M.; Arkas, M.; Skoulios, A. Mesomorphic Character of Quaternary Ammonium Salts Affected by Secondary Hydrogen Bonding Interactions. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1998, 309, 237–250. [Google Scholar] [CrossRef]
- Murugan, E.; Arumugam, S.; Panneerselvam, P. New nanohybrids from poly(propylene imine) dendrimer stabilized silver nanoparticles on multiwalled carbon nanotubes for effective catalytic and antimicrobial applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 65, 111–124. [Google Scholar] [CrossRef]
- Nemanashi, M.; Meijboom, R. “Cat in a bag” recycling of dendrimer encapsulated Au nanoparticles by use of dialysis membrane bag in the reduction of 4-nitrophenol: Proof of heterogeneous catalysis. Catal. Commun. 2016, 83, 53–57. [Google Scholar] [CrossRef]
- Pan, H.; Liu, D.; Hu, N.; Shi, J.; Liu, H.-X. Hyperbranched polymer-protected gold nanoparticles well-dispersed in different organic solvents: Preparation and their catalytic applications to 4-nitrophenol reduction. Colloid Polym. Sci. 2015, 293, 2017–2026. [Google Scholar] [CrossRef]
- Liu, H.; Wan, D.; Du, J.; Jin, M. Dendritic Amphiphile Mediated One-Pot Preparation of 3D Pt Nanoparticles-Decorated PolyHIPE as a Durable and Well-Recyclable Catalyst. ACS Appl. Mater. Interfaces 2015, 7, 20885–20892. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Feng, Y.; Wan, D.; Jin, M. Polyamino amphiphile mediated support of platinum nanoparticles on polyHIPE as an over 1500-time recyclable catalyst. RSC Adv. 2016, 6, 109253–109258. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, M.; Wan, D. One-pot synthesis of porous monolith-supported gold nanoparticles as an effective recyclable catalyst. J. Mater. Chem. A 2015, 3, 13519–13525. [Google Scholar] [CrossRef]
- Rajesh, R.; Kumar, S.S.; Venkatesan, R. Efficient degradation of azo dyes using Ag and Au nanoparticles stabilized on graphene oxide functionalized with PAMAM dendrimers. New J. Chem. 2014, 38, 1551–1558. [Google Scholar] [CrossRef]
- Hao, B.; Lu, G.; Zhang, S.; Li, Y.; Ding, A.; Huang, X. Gold nanoparticles standing on PEG/PAMAM/thiol-functionalized nanographene oxide as aqueous catalysts. Polym. Chem. 2020, 11, 4094–4104. [Google Scholar] [CrossRef]
- Hassan, N.; Shahat, A.; El-Didamony, A.; El-Desouky, M.; El-Bindary, A. Mesoporous iron oxide nano spheres for capturing organic dyes from water sources. J. Mol. Struct. 2020, 1217, 128361. [Google Scholar] [CrossRef]
- Ilunga, A.K.; Meijboom, R. Random alloy nanoparticles of Pd and Au immobilized on reducible metal oxides and their catalytic investigation. Appl. Catal. B Environ. 2017, 203, 505–514. [Google Scholar] [CrossRef]
- Xaba, M.S.; Noh, J.-H.; Meijboom, R. Catalytic activity of different sizes of Pt/Co3O4 in the oxidative degradation of Methylene Blue with H2O2. Appl. Surf. Sci. 2018, 467–468, 868–880. [Google Scholar] [CrossRef]
- Ramaraju, B.; Imae, T.; Destaye, A.G. Ag nanoparticle-immobilized cellulose nanofibril films for environmental conservation. Appl. Catal. A Gen. 2015, 492, 184–189. [Google Scholar] [CrossRef]
- Nabil, B.; Morshed, M.N.; Ahmida, E.A.; Nemeshwaree, B.; Christine, C.; Julien, V.; Olivier, T.; Abdelkrim, A. Development of new multifunctional filter based nonwovens for organics pollutants reduction and detoxification: High catalytic and antibacterial activities. Chem. Eng. J. 2018, 356, 702–716. [Google Scholar] [CrossRef]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Guan, J.; Nierstrasz, V. Stabilization of zero valent iron (Fe0) on plasma/dendrimer functionalized polyester fabrics for Fenton-like removal of hazardous water pollutants. Chem. Eng. J. 2019, 374, 658–673. [Google Scholar] [CrossRef]
- Morshed, M.N.; Miankafshe, M.A.; Persson, N.-K.; Behary, N.; Nierstrasz, V.A. Development of a multifunctional graphene/Fe-loaded polyester textile: Robust electrical and catalytic properties. Dalton Trans. 2020, 49, 17281–17300. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Bouazizi, N.; Behary, N.; Vieillard, J.; Thoumire, O.; Nierstrasz, V.; Azzouz, A. Iron-loaded amine/thiol functionalized polyester fibers with high catalytic activities: A comparative study. Dalton Trans. 2019, 48, 8384–8399. [Google Scholar] [CrossRef] [PubMed]
- Murugan, E.; Shanmugam, P. Surface grafted hyper-branched polyglycerol stabilized Ag and AuNPs heterogeneous catalysts for efficient reduction of Congo red. J. Nanosci. Nanotechnol. 2016, 16, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P.; Rajakumar, K.; Boddula, R.; Ngullie, R.C.; Wei, W.; Xie, J.; Murugan, E. Heterogeneous form of poly (4-vinyl pyridine) beads based dendrimer stabilized Ag, Au and PdNPs catalyst for reduction of trypan blue. Mater. Sci. Energy Technol. 2019, 2, 532–542. [Google Scholar] [CrossRef]
- Murugan, E.; Jebaranjitham, J.N.; Raman, K.J.; Mandal, A.; Geethalakshmi, D.; Kumar, M.D.; Saravanakumar, A. Insoluble dendrimer-grafted poly(vinylimidazole) microbeads stabilized with mono/bimetallic nanoparticle catalysts for effective degradation of malachite green. New J. Chem. 2017, 41, 10860–10871. [Google Scholar] [CrossRef]
- Nemanashi-Maumela, M.; Nongwe, I.; Motene, R.C.; Davids, B.L.; Meijboom, R. Au and Ag nanoparticles encapsulated within silica nanospheres using dendrimers as dual templating agent and their catalytic activity. Mol. Catal. 2017, 438, 184–196. [Google Scholar] [CrossRef]
- Arkas, M.; Kithreoti, G.; Boukos, N.; Kitsou, I.; Petrakli, F.; Panagiotaki, K. Two completely different biomimetic reactions mediated by the same matrix producing inorganic/organic/inorganic hybrid nanoparticles. Nano-Struct. Nano-Objects 2018, 14, 138–148. [Google Scholar] [CrossRef]
- Onisuru, O.R.; Oseghale, C.O.; Meijboom, R. In situ replacement of Cu-DEN: An approach for preparing a more noble metal nanocatalyst for catalytic use. New J. Chem. 2020, 44, 20322–20333. [Google Scholar] [CrossRef]
- Murugan, E.; Jebaranjitham, J.N. Dendrimer grafted core–shell Fe3O4–polymer magnetic nanocomposites stabilized with AuNPs for enhanced catalytic degradation of Rhodamine B—A kinetic study. Chem. Eng. J. 2015, 259, 266–276. [Google Scholar] [CrossRef]
- Malinga, S.P.; Arotiba, O.; Krause, R.W.M.; Mapolie, S.F.; Diallo, M.S.; Mamba, B.B. Composite polyester membranes with embedded dendrimer hosts and bimetallic Fe/Ni nanoparticles: Synthesis, characterisation and application to water treatment. In Nanotechnology for Sustainable Development; Springer: Cham, Switzerland, 2013; pp. 47–61. [Google Scholar] [CrossRef]
- Sivasankar, V.; Nkonde, M.A.; Govender, P.; Omine, K.; Kuvarega, A.T.; Prabhakaran, M.; Msagati, T.A. Dendrimer supported Fe/Ni bimetallic composites immobilized in polyethersulfone membranes for effective degradation of arginine containing microcystins. Eur. Polym. J. 2018, 98, 456–467. [Google Scholar] [CrossRef]
- Vlotman, D.; Ngila, J.; Ndlovu, T.; Doyle, B.; Carleschi, E.; Malinga, S. Hyperbranched polymer membrane for catalytic degradation of polychlorinated biphenyl-153 (PCB-153) in water. React. Funct. Polym. 2018, 136, 44–57. [Google Scholar] [CrossRef]
- Wang, X.X.; Chen, T.; Wang, J.P. Degradation of Synthetic Reactive Dye Wastewater Based on Amine-Functionalized Polyacrylonitrile Fiber. Key Eng. Mater. 2015, 671, 425–430. [Google Scholar] [CrossRef]
- Aldana, A.A.; Strumia, M.C.; Martinelli, M. The Cooperative Effect in Dendronized Chitosan Microbeads. Aust. J. Chem. 2015, 68, 1918. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.A.; Darwish, A.S.; Gobara, H.; Abed-Elsatar, N.E.; Fouda, S.R. Interaction profiles in poly (amidoamine) dendrimer/montmorillonite or rice straw ash hybrids-immobilized magnetite nanoparticles governing their removal efficiencies of various pollutants in wastewater. J. Mol. Liq. 2017, 230, 353–369. [Google Scholar] [CrossRef]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- Kitsou, I.; Arkas, M.; Tsetsekou, A. Synthesis and characterization of ceria-coated silica nanospheres: Their application in heterogeneous catalysis of organic pollutants. SN Appl. Sci. 2019, 1, 1557. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Ni, H.; Wang, L.; Wu, M.; Yang, X. Nanoscale-confined precursor of CuFe2O4 mediated by hyperbranched polyamide as an unusual heterogeneous Fenton catalyst for efficient dye degradation. J. Clean. Prod. 2018, 186, 146–154. [Google Scholar] [CrossRef]
- Kabel, K.I.; Mady, A.H.; Rabie, A.M. Novel preparation of ferromanganese oxide based on hyperbranched polymer for peroxymonosulfate activation as a robust catalyst for the degradation of organic pollutants. Environ. Technol. Innov. 2021, 22, 101435. [Google Scholar] [CrossRef]
- Brazkova, M.; Gerginova, M.; Gitsov, I.; Krastanov, A. Microbes in the Spotlight: Recent Progress in the Understanding of Beneficial and Harmful Microorganisms; Mendez-Vilas, A., Ed.; Brown Walker Press: Boca Raton, FL, USA, 2016; pp. 45–51. [Google Scholar]
- Soozanipour, A.; Taheri-Kafrani, A.; Razmjou, A.; Asadnia, M. Hyaluronidase enzyme conjugated polyamidoamine dendrimer: An efficient and stable nanobiocatalyst for enzymatic degradation of hyaluronic acid. J. Mol. Liq. 2021, 118111. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Kiwaan, H.; Atwee, T.; Azab, E.; El-Bindary, A. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide. J. Mol. Struct. 2019, 1200, 127115. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Imae, T. Synthesis of dendrimer-protected TiO2 nanoparticles and photodegradation of organic molecules in an aqueous nanoparticle suspension. J. Colloid Interface Sci. 2005, 285, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Imae, T. Preparation of siloxy focal dendron-protected TiO2 nanoparticles and their photocatalysis. J. Colloid Interface Sci. 2006, 297, 122–129. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, C.; Lei, H.; Huo, J. Visible light photocatalytic activity of aromatic polyamide dendrimer/TiO2 composites functionalized with spirolactam-based molecular switch. J. Colloid Interface Sci. 2013, 406, 178–185. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Badawy, A.A.; Ismail, N.; Tian, Z.R.; Rehim, M.A.; Rabia, A. Photocatalytic activity of hyperbranched polyester/TiO2 nanocomposites. Appl. Catal. A Gen. 2014, 472, 191–197. [Google Scholar] [CrossRef]
- Kim, L.-J.; Jang, J.-W.; Park, J.-W. Nano TiO2-functionalized magnetic-cored dendrimer as a photocatalyst. Appl. Catal. B Environ. 2014, 147, 973–979. [Google Scholar] [CrossRef]
- Jung, J.-J.; Jang, J.-W.; Park, J.-W. Effect of generation growth on photocatalytic activity of nano TiO2-magnetic cored dendrimers. J. Ind. Eng. Chem. 2016, 44, 52–59. [Google Scholar] [CrossRef]
- Mahmood, A.; Park, J.-W. TiO2/CdS nanocomposite stabilized on a magnetic-cored dendrimer for enhanced photocatalytic activity and reusability. J. Colloid Interface Sci. 2019, 555, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.R.; Huang, B.Q.; Chen, Q.H.; Xue, H.; Qian, Q.R.; Xiao, L.R.; Liu, X.P.; Xu, J. Photocatalysis Activity of TiO2-Based/Dendrimer Phthalocyanine Nanocomposite Photocatalyst. Appl. Mech. Mater. 2014, 703, 86–89. [Google Scholar] [CrossRef]
- Monzavi, A.; Montazer, M.; Malek, R.M.A. A Novel Polyester Fabric Treated with Nanoclay/Nano TiO2/PAMAM for Discoloration of Reactive Red 4 from Aqueous Solution Under UVA Irradiation. J. Polym. Environ. 2016, 25, 1321–1334. [Google Scholar] [CrossRef]
- Nzaba, S.K.M.; Nyoni, H.; Mamba, B.; Kuvarega, A.T. Comparative Study of Dendrimer-Templated Nitrogen-Platinum Co–Doped TiO2 for the Photocatalytic Degradation of Azo Dyes in Contaminated Water. ChemistrySelect 2019, 4, 12156–12163. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Karousis, N.; Zhao, S.; Wang, Q.; Shinohara, H.; Tagmatarchis, N. Photocatalytic application of nanosized CdS immobilized onto functionalized MWCNTs. Dalton Trans. 2014, 43, 7429–7434. [Google Scholar] [CrossRef]
- Wang, Q.; Xiang, Y.; Li, X.; Zhang, W.; Huang, X.; Qian, X. Stable construction of layered reduced grapheme oxide/copper sulfide composites on cellulose fibers with hyperbranched polyamide-amine for efficient photocatalytic degradation of organic dyes. Ind. Crop. Prod. 2021, 170, 113695. [Google Scholar] [CrossRef]
- Mousavi, S.; Shahraki, F.; Aliabadi, M.; Haji, A.; Deuber, F.; Adlhart, C. Nanofiber immobilized CeO2/dendrimer nanoparticles: An efficient photocatalyst in the visible and the UV. Appl. Surf. Sci. 2019, 479, 608–618. [Google Scholar] [CrossRef]
- Kutz, A.; Mariani, G.; Schweins, R.; Streb, C.; Gröhn, F. Self-assembled polyoxometalate–dendrimer structures for selective photocatalysis. Nanoscale 2017, 10, 914–920. [Google Scholar] [CrossRef]
- Alfano, B.; Barretta, L.; Del Giudice, A.; De Vito, S.; Di Francia, G.; Esposito, E.; Formisano, F.; Massera, E.; Miglietta, M.L.; Polichetti, T. A Review of Low-Cost Particulate Matter Sensors from the Depvelopers’ Perspectives. Sensors 2020, 20, 6819. [Google Scholar] [CrossRef]
- Shaku, K.M.; Dlamini, L.N.; Malinga, S.P. Highly efficient photocatalytic hyperbranched polyethyleneimine/bismuth vanadate membranes for the degradation of triclosan. Int. J. Environ. Sci. Technol. 2020, 17, 3297–3312. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, B.; Cong, Q.; Ma, L.W.; He, X.C.; Gao, M.J.; Bian, L.; Ma, X.F.; Li, G. Surface Modification of Low-Dimensional Heterostructured Functional Materials with Dendrimers and their Properties of Organic-Inorganic Nanocomposites. In Materials Science Forum; Trans Tech Publications Ltd.: Baech, Switzerland, 2016; Volume 847, pp. 299–307. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Badawy, A.A.; Mohram, M.E.; Rehim, M.A. Enhancement the Photocatalytic and Biological Activity of Nano-sized ZnO Using Hyperbranched Polyester. J. Inorg. Organomet. Polym. Mater. 2019, 29, 928–938. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Imae, T. Chemically modified novel PAMAM-ZnO nanocomposite: Synthesis, characterization and photocatalytic activity. Appl. Catal. A Gen. 2014, 486, 170–175. [Google Scholar] [CrossRef]
- Duarah, R.; Karak, N. Hyperbranched polyurethane/reduced carbon dot-zinc oxide nanocomposite-mediated solar-assisted photocatalytic degradation of organic contaminant: An approach towards environmental remediation. Chem. Eng. J. 2019, 370, 716–728. [Google Scholar] [CrossRef]
- Hassan, N.; Shahat, A.; El-Didamony, A.; El-Desouky, M.; El-Bindary, A. Synthesis and characterization of ZnO nanoparticles via zeolitic imidazolate framework-8 and its application for removal of dyes. J. Mol. Struct. 2020, 1210, 128029. [Google Scholar] [CrossRef]
- Yang, J.; Chu, S.; Guo, Y.; Luo, L.; Kong, F.; Wang, Y.; Zou, Z. Hyperbranched polymeric N-oxide: A novel kind of metal-free photocatalyst. Chem. Commun. 2012, 48, 3533–3535. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Synthesis, characterization, catalytic, DNA binding and antibacterial activities of Co(II), Ni(II) and Cu(II) complexes with new Schiff base ligand. J. Mol. Liq. 2021, 326, 115223. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Structural characterization and biological activity of a new metal complexes based of Schiff base. J. Mol. Liq. 2021, 330, 115522. [Google Scholar] [CrossRef]


















| Dendritic Polymer | Metal Nanoparticles | Reference |
|---|---|---|
| PAMAM G3, G5 PPI G3, G4 | Au | [69] |
| PAMAM G3, G4, G5, PPI G2, G3, G4 | Ag, Pt, Pd | [70] |
| PAMAM G3, G3.5, G5, G5.5 | Ag, Au Ag/Au alloy | [79] |
| PAMAM-OH G6 PAMAM G4 | Au, Cu, Pd, Pt, Au/Cu, Pd/Cu, Pt/Cu | [80] |
| PAMAM G4 | Cu | [72] |
| PAMAM-OH G4 | Ru, Ni, Ru/Ni | [81] |
| [PEG-G1-(3,5-DHB-OH)16] | Ag | [93] |
| PEI core 2,2-Bis(hydroxymethyl)propionic acid dendrons | Au | [96] |
| Hyperbranched Polystyrene | Au | [97] |
| PAMAM-OH G4, G5, G6 | Ru | [81] |
| PAMAM-OH, PAMAM-NH2 G4, G6 | Cu, Ag, Au | [74] |
| PAMAM G4 PAMAM-OH G4, G6 | Pd | [73] |
| Glycodendrimers | Pd | [84] |
| Glycodendrimers | Au | [83] |
| PAMAM-OH G4, G5, G6 | Pd | [75] |
| PAMAM-OH G4, G5 | Pd | [71] |
| Amphiphilic PPI-G2 | Ru | [95] |
| PAMAM G4 | Ag, Au | [76] |
| Nona-PEG-branched Triazole Dendrimers | Au | [85] |
| Glycodendrimers G1-G4 | Au | [88] |
| PAMAM-OH G4, G5, G6 | Pd, Pt | [78] |
| PEI core amide shells | Au | [98] |
| Hyperbranched Polyester Boltron-PEI-PEG | Au | [103] |
| Linear PEG-Hyperbranched PEI poly(ε-caprolactone) | Pt | [105] |
| Bistriazole-based dendritic amphiphile micelles | Ag, Cu | [89] |
| PAMAM G5-maleic anhydride-cysteamine | Au | [100] |
| Triethylene glycol-Arene-Triazole click Dendrimers | Fe, Co, Ni, Cu, Ru, Rh, Pd, Ag. Ir, Pt, Au | [86] |
| Amine-modified hyperbranched polyester PEG copolymer | Au | [104] |
| PAMAM-OH G4, G5, G6 | Cu | [77] |
| Jeffamine core PAMAM G4 | Ag | [94] |
| 1,2,3-Triazolyl Dendronized Polymers | Au | [90] |
| Ferrocenyl/Triethylele glycol Dendronized Polymers | Au | [91] |
| Ferrocenyl/Triethylele glycol Dendronized Diblock Polymers | Au, Ag | [92] |
| 4-Carbomethoxypyrrolidone PAMAM G3-G5 | Ag | [101] |
| Triazine-based dendrimers | Ir, Pt | [99] |
| Dendritic Polymer | Metal NPs | Pollutant | Degradation Products | Ref. |
|---|---|---|---|---|
| Polyaryl ether trisacetic acid ammonium chloride dendrons | Pt | p-nitrophenol, o-nitroanisole, o-,m-,p-nitrotoluene | p-nitroaniline, o-anisidine and o-,m-,p-aminotoluene | [106] |
| Polyaryl ether trisacetic acid ammonium chloride dendrons | Au/Pt | p-nitrophenol, o-nitroanisole, o-,m-,p-nitrotoluene, 3-phenoxybenzaldehyde | p-nitroaniline, o-anisidine and o-,m-,p-aminotoluene, 3-phenoxyphenyl methanol | [107] |
| Amphiphilic PPI-G2 | Au | Nitrobenzene | Aniline | [108] |
| PEG core dendrimer | Ag | 4-nitrobenzaldehyde, nitrobenzene, 4-nitrotoluene, 4-nitroaniline. 4-nitrocatechol, 2-hydroxy-5- nitrobenzyl bromide, 5-hydroxy-2-nitrobenzaldehyde | 4-aminobenzaldehyde, aniline, 4-anisidine, 4-phenylalanine. 4-aminocatechol, 2-hydroxy-5-aminobenzyl bromide, 5-hydroxy-2-aminobenzaldehyde | [109] |
| PEG-PAMAM G3 | Cu | 4-(4-nitrophenyl) morpholine, 4-(2-fluoro-4- nitrophenyl) morpholine), 4-(4-nitrophenyl) morpholin-3-one | 4-(4-aminophenyl) morpholine, 4-(2-fluoro-4-aminophenyl) morpholine), 4-(4-aminophenyl) morpholin-3-one | [110] |
| PEG-PAMAM G3 | Au | 4-(4-nitrophenyl) morpholine, 4-(2-fluoro-4- nitrophenyl) morpholine), 4-(4-nitrophenyl) morpholin-3-one | 4-(4-aminophenyl) morpholine, 4-(2-fluoro-4-aminophenyl) morpholine), 4-(4-aminophenyl) morpholin-3-one | [111] |
| PAMAM G2 | Cu | 4-nitrophenol, 2-nitrophenol, 4-nitrobenzaldehyde, 2,4 dinitrophenol, 2-nitroaniline, 4-nitroaniline, 3-nitrotoluene, 4-nitrotoluene, 4-nitrochlorobenzene | 4-aminophenol, 2-aminophenol, 4-amino benzaldehyde, 2-nitro-4-aminophenol, 2-phenyl diamine, 4-phenyl diamine, 3-aminotoluene, 4-aminotoluene, 4-aminochlorobenzene | [112] |
| Dendritic Polymer | Metal NPs | Dye | Degradation Products | Reference |
|---|---|---|---|---|
| Octyl PPI-G2 | Ag, Pd, Pt | Methyl Orange |
| [113] |
| PAMAM-OH G6 | Pd, Pt | Morin |
| [117] |
| G6-PAMAM-NH2 | Au | Morin |
| [116] |
| PAMAM PAMAM-OH G4, G5 | Pd, Au | Methylene Blue | Leuko-methylene blue | [122] |
| PAMAM G5 | Au, Ag | Methylene blue |
| [121] |
| PAMAM G5 | Au, Ag | Morin |
| [115] |
| PAMAM G6 | Pd/Au | Morin |
| [118] |
| PAMAM-OH G6 | Pd/Au | Methyl Orange |
| [114] |
| PAMAM G5 functionalized by maleic anhydride and cysteamine | Pd | Morin |
| [120] |
| PAMAM G5 functionalized by maleic anhydride and cysteamine | Pd | Morin |
| [119] |
| Dendritic Polymer | Metal NPs | Substrate-Formulation | Reference |
|---|---|---|---|
| PPI-G2 | Au | Poly(4-vinyl pyridine) beads | [123] |
| PAMAM G4 Dendrons | Pt | SBA-15 SiO2 | [128] |
| PAMAM G5 Dendrons | Ag | Polystyrene microsphere core SiO2 shells | [124] |
| PAMAM G7 Dendrons | Ag | Fe3O4 coated by polystyrene | [130] |
| PAMAM G3 Dendrons | Ag | Graphite | [135] |
| PEI | Au | Polyacrylonitrile fiber | [126] |
| Amphiphilic PPI G2, G3 | Ag, Pd | MWCNT | [137] |
| PAMAM G1 | Ag | Fe3O4 | [131] |
| PAMAM G2 | Au | Polyacrylic acid/polyvinyl alcohol nanofibers | [127] |
| PEI Core Polystyrene dodecyl shell | Au | Polymer open-cellular elastic monolith | |
| PEI Core Poly(styrene-co-2-ethyl hexyl acrylate shell. | Pt | Copolymer with 2-ethylhexyl acrylate-poly(ethylene glycol) dimethacrylate open-cellular elastic monolith | [142] |
| Amphiphilic PEI | Au | Chloroform, toluene, or petroleum ether | [141] |
| PEI Core | Au | Polystyrene dodecyl shell | [144] |
| PAMAM G6 | Au | Cellulose nanocrystals | [125] |
| PAMAM-G4 | Au/Ag | Glass microreactors | [129] |
| PPI G2 | Ag | MWCNT | [139] |
| PEI Core poly(styrene-co-2-Ethylhexyl acrylate) Shell | Pt | Copolymerization with 2-ethylhexyl acrylate-poly(ethylene glycol) dimethacrylate | [143] |
| PAMAM G4 | Au | Dialysis membrane | [140] |
| SiO2 Dendrons | Ag | Fe3O4@SiO2@dendritic-SiO2-NH2-Ag | [133] |
| PAMAM G2 Dendrons | Au | Fe3O4, KH-570 glycidyl methacrylate divinylbenzene copolymer, | [132] |
| PAMAM G3 dendrons | Pd | Graphene oxide, MnFe2O4 NPs | [136] |
| Dendritic Polymer | Metal NPs | Substrate-Formulation | Dyes | Reference |
|---|---|---|---|---|
| PAMAM Dendrons | Ag/Au | GO | Methyl orange, Congo red | [145] |
| PAMAM G4 | Ag | Cellulose nanofibril films | Rhodamine B | [150] |
| PAMAM Dendrons G0, G1, G2 | Au | Fe3O4 core 4-methyl styrene-divinylbenzene glycidyl methacrylate shell beads | Rhodamine B | [161] |
| Hyperbranched PG | Ag, Au | Poly(styrene)-co-poly(vinyl benzene chloride) beads | Congo Red | [155] |
| PAMAM G4 | Ag, Au | SiO2 nanospheres | Methylene blue | [158] |
| PAMAM-OH G5 | Pd/Au | CeO2, NiO, Fe2O3, MnO2, SiO2, Co3O4 | Morin | [148] |
| PPI G2, G3 | Au, Au/Pd | Poly(vinyl imidazole) microbeads | Malachite green | [157] |
| PPI(G2) and PPI(G3) | Ag, Au, Pd | 4-Vinyl pyridine) beads | Trypan blue | [156] |
| PAMAM | Cu, Ag | Polyester nonwoven fabrics | 4-Nitrophenol, methylene blue, malachite green, remazol red | [151] |
| PAMAM-OH G6 | Pt | Mesoporous Co3O4 | Methylene blue | [149] |
| PAMAM G1 | Fe | Polyester fabrics | 4-Nitrophenol, methylene blue | [154] |
| PEG | Fe | Fibrous polyester membrane | Malachite green | [152] |
| PAMAM | Fe | GO, polyester textile | Crystal violet | [153] |
| PAMAM, PEG dendrigraft | Au | Nanographene oxide | 4-Nitrophenol, 4-nitroaniline Congo red | [146] |
| PAMAM G4 | Au | SiO2 nanospheres | 4-Nitrophenol, rhodamine B | [160] |
| Dendritic Polymer | Active Ingredient | Substrate-Formulation | Pollutants | Reference |
|---|---|---|---|---|
| Hexa and nona-functionalized amido dendrons | Cu2+ | Chitosan microbeads | Organic water and soil pollutants | [166] |
| PEI | Fe3+ | Polyacrylonitrile fiber | Reactive red 195 | [165] |
| Dendritic linear dendritic copolymer polybenzyl ether G2, G3, G4-PEG5000-G2 | Laccase | - | Bisphenol A | [172] |
| PAMAM G3 | Fe3O4 | Montmorillonite or rice-straw-ash | Malachite green, xylenol orange | [167] |
| PEI | SiO2@TiO2 core-shell nanospheres | - | 4-Nitrophenol | [168] |
| PEI | CeO2@SiO2 core-shell nanospheres | - | 4-Nitrophenol | [169] |
| Hyperbranched polyamide | CuFe2O4 | - | Reactive red 2, reactive yellow 3, basic red 46, basic yellow 24 | [170] |
| Hyperbranched phenylene diamine methyl methacrylate | FeMnO3 | - | Methylene blue, tetracycline, Rhodamine B | [171] |
| PAMAM G4 | Hyaluronidase | - | Hyaluronic acid | [173] |
| Dendritic Polymer | Photocatalyst/Substrate | Pollutants | Reference |
|---|---|---|---|
| PAMAM-NH2 G4 PAMAM-OH G4 PAMAM-COONa G4 | TiO2 | 2,4-Dichlorophenoxyacetic acid | [176] |
| Alkylated PAMAM G1, G2, G3 Dendrons | TiO2@SiO2 core-shell | 2,4-Dichlorophenoxyacetic acid | [177] |
| Hyperbranched polyimide N-oxide (PINO) | - | Methyl orange | [197] |
| Aromatic Polyamide Dendrimer Functionalized with Spirolactam | TiO2 | Phenol | [178] |
| Hyperbranched polyester HPES-OH | ΤιO2 nanowires | Wastewater | [179] |
| PAMAM dendrons | Fe3O4, TiO2, | Methyl orange | [180] |
| Poly (aryl benzyl ether) Zn phthalocyanine Dendrimer | Polycrystalline TiO2 | Rhodamine B | [183] |
| PAMAM Dendron | CdS | Rhodamine B | [186] |
| PAMAM G4 | ZnO | Naphthol blue black | [194] |
| PAMAM G4 | TiO2/nanoclay polyester Fabric | Reactive red 4 | [184] |
| PAMAM dendrons | Fe3O4, TiO2 | Methyl orange | [181] |
| PAMAM G2.5 | ZnO/CuS low-dimensional heterostructured composites | Rhodamine B, methylene blue | [192] |
| PAMAM G4 | K4 [SiW12O40] (POM) | Methyl Red, methylene blue, alizarin yellow R, xylenol orange | [189] |
| PAMAM G0, G1, PEI | Pt TiO2 | Brilliant black | [185] |
| PAMAM G0, G1 Dendrons | TiO2, CdS, Fe3O4 | Methyl orange | [182] |
| Hyperbranched Polyester | ZnO | (N-(1-naphthyl)-ethylene diamine dichloride, catechol, sulfanilamide | [193] |
| Hyperbranched polyurethane | Reduced carbon dot-ZnO2 | Dodecyl-benzenesulfonate, Commercial detergent | [195] |
| PAMAM G3 | CeO2 pullulan/poly(vinyl alcohol)/poly(acrylic acid) nanofibers | Phenol, 4-hydroxy-1-naphthalene sulfonic acid sodium salt, and azorubine dye | [188] |
| PEI | BiVO4 | Triclosan (5-chloro-2-(2,4-dichloro phenoxy)phenol) | [191] |
| Hyperbranched PAMAM | CuS reduced GO, dDialdehyde cellulose fiber | Rhodamine B | [187] |
| PAMAM | MoS2 | Chlorpyrifos, glyphosate | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers. Nanomaterials 2022, 12, 445. https://doi.org/10.3390/nano12030445
Arkas M, Anastopoulos I, Giannakoudakis DA, Pashalidis I, Katsika T, Nikoli E, Panagiotopoulos R, Fotopoulou A, Vardavoulias M, Douloudi M. Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers. Nanomaterials. 2022; 12(3):445. https://doi.org/10.3390/nano12030445
Chicago/Turabian StyleArkas, Michael, Ioannis Anastopoulos, Dimitrios A. Giannakoudakis, Ioannis Pashalidis, Theodora Katsika, Eleni Nikoli, Rafael Panagiotopoulos, Anna Fotopoulou, Michail Vardavoulias, and Marilina Douloudi. 2022. "Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers" Nanomaterials 12, no. 3: 445. https://doi.org/10.3390/nano12030445