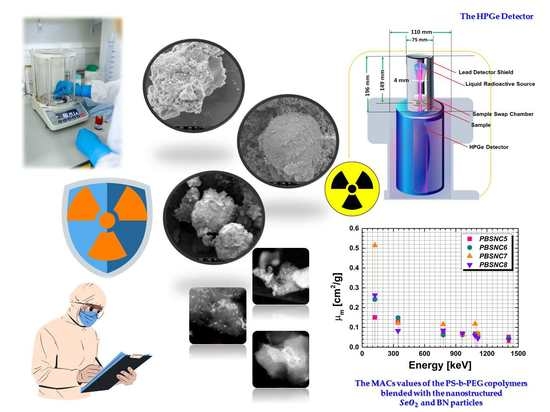
Radiation Shielding Tests of Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers Blended with Nanostructured Selenium Dioxide and Boron Nitride Particles
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis and Characterization
2.2. Characterization of Polymer–Nanostructured-Particle-Based Nanocomposites
2.2.1. Thermogravimetric Analysis (TGA)
2.2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Examinations
2.2.3. Transmission Electron Microscopy (TEM) and EDX Examinations
2.3. Preparation of Polymer–Nanostructured-Particle-Based Nanocomposites
2.4. Experimental Framework for Gamma Irradiation of Polymer–Nanostructured-Particle-Based Nanocomposites
3. Results and Discussions
3.1. Characterization of Polymer–Nanostructured-Particle-Based Nanocomposites via SEM, TEM, and EDX Systems
3.1.1. Characterization of Polymer–Nanostructured-Particle-Based Nanocomposites via SEM
3.1.2. Characterization of Polymer–Nanostructured-Particle-Based Nanocomposites via TEM
3.2. Thermogravimetric Analysis (TGA) Outcomes of the PS-b-PEG Copolymers Blended with the Nanostructured SeO2 and BN Particles
3.3. Gamma Irradiation Results
3.3.1. Linear () and Mass Attenuation () Coefficients
3.3.2. Half-Value Layer (HVL), Tenth Value Layer (TVL), Mean Free Path (MFP), and Radiation Protection Efficiency (RPE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemeda, O.M.; Eid, M.E.A.; Sharshar, T.; Ellabany, H.M.; Henaish, A.M.A. Synthesis of nanometer-sized PbZrxTi1−xO3 for gamma-ray attenuation. J. Phys. Chem. Solids 2021, 148, 109688. [Google Scholar] [CrossRef]
- Moharram, B.M.; Nagy, M.E.; Shaat, M.K.; El Sayed, A.R.; Fayiz, M.; Dwidar, S.A.; Dorrah, M.E. Performance of lead and iron oxides nanoparticle materials on shielding properties for γ-rays. Radiat. Phys. Chem. 2020, 173, 108880. [Google Scholar] [CrossRef]
- Ban, C.C.; Khalaf, M.A.; Ramli, M.; Ahmed, N.M.; Abunahel, B.M.; Dawood, E.T.; Ameri, F. Effect of nano-silica slurry on engineering, X-ray, and γ-ray attenuation characteristics of steel slag high-strength heavyweight concrete. Nanotechnol. Rev. 2020, 9, 1245–1264. [Google Scholar] [CrossRef]
- Asgari, M.; Afarideh, H.; Ghafoorifard, H.; Amirabadi, E.A. Comparison of nano/micro lead, bismuth and tungsten on the gamma shielding properties of the flexible composites against photon in wide energy range (40 keV–662 keV). Nucl. Eng. Technol. 2021, 53, 4142–4149. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Ashry, A.; El-Taher, A.; Abbady, A.G.E.; Allam, E.A.; El-Sharkawy, R.M.; Mahmoud, M.E. Role of novel ternary nanocomposites polypropylene in nuclear radiation attenuation properties: In-depth simulation study. Radiat. Phys. Chem. 2021, 188, 109667. [Google Scholar] [CrossRef]
- Caglar, M.; Karabul, Y.; Kılıc, M.; Ozdemir, Z.G.; Icelli, O. Na2Si3O7/Ag micro and nano-structured glassy composites: The experimental and MCNP simulation surveys of their radiation shielding performances. Prog. Nucl. Energy 2021, 139, 103855. [Google Scholar] [CrossRef]
- Kadhim, M.A.; Al-Bermany, E. New fabricated PMMA-PVA/graphene oxide nanocomposites: Structure, optical properties and application. J. Compos. Mater. 2021, 55, 2793–2806. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; Kassem, S.M.; Bekhit, M.; Fahim, R.A.; Ashour, A.H.; Awed, A.S. Gamma radiation shielding properties of poly(vinyl butyral)/Bi2O3@BaZrO3 nanocomposites. Mater. Chem. Phys. 2021, 268, 124728. [Google Scholar] [CrossRef]
- El-Taher, A.; Zakaly, H.M.H.; Pyshkina, M.; Allam, E.A.; El-Sharkawy, R.M.; Mahmoud, M.E.; Abdel-Rahman, M.A.E. A comparative Study Between Fluka and Microshield Modeling Calculations to study the Radiation-Shielding of Nanoparticles and Plastic Waste composites. Z. Anorg. Allg. Chem. 2021, 647, 1083. [Google Scholar] [CrossRef]
- Mansouri, E.; Mesbahi, A.; Malekzadeh, R.; Janghjoo, A.G.; Okutan, M. A review on neutron shielding performance of nanocomposite materials. Int. J. Radiat. Res. 2020, 18, 611–622. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Baskan, T.; Erol, B.; Mutlu, S.; Misirlioglu, Y.; Savaskan Yilmaz, S.; Yilmaz, A.H. Gamma irradiation, thermal conductivity, and phase change tests of the cement-hyperbranched poly amino-ester-block-poly cabrolactone-polyurathane plaster-lead oxide and arsenic oxide composite for development of radiation shielding material. Int. J. Energy Res. 2021, 45, 20729–20762. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Erol, B.; Baskan, T.; Mutlu, S.; Savaskan Yilmaz, S.; Yilmaz, A.H. Gamma Irradiation and the Radiation Shielding Characteristics: For the Lead Oxide Doped the Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers and the Polystyrene-b-Polyethyleneglycol-Boron Nitride Nanocomposites. Polymers 2021, 13, 3246. [Google Scholar] [CrossRef] [PubMed]
- Castley, D.; Goodwin, C.; Liu, J. Computational and experimental comparison of boron carbide, gadolinium oxide, samarium oxide, and graphene platelets as additives for a neutron shield. Radiat. Phys. Chem. 2019, 165, 108435. [Google Scholar] [CrossRef]
- Basu, P.; Sarangapani, R.; Venkatraman, B. Compact shielding design for 740 GBq 241Am-Be neutron source transport container. Radiat. Phys. Chem. 2020, 170, 108670. [Google Scholar] [CrossRef]
- Hu, G.; Hu, H.; Yang, Q.; Yu, B.; Sun, W. Study on the design and experimental verification of multilayer radiation shield against mixed neutrons and γ-rays. Nucl. Eng. Technol. 2020, 52, 178–184. [Google Scholar] [CrossRef]
- Shahri, K.K.; Motavalli, L.R.; Hakimabad, H.M. Finding a suitable shield for mixed neutron and photon fields based on an Am–Be source. J. Radioanal. Nucl. Chem. 2013, 298, 33–39. [Google Scholar] [CrossRef]
- Tekin, H.O.; Issa, S.A.M.; Kilic, G.; Zakaly, H.M.H.; Tarhan, N.; Sidek, H.A.A.; Matori, K.A.; Zaid, M.H.M. A Systematical Characterization of TeO2–V2O5 Glass System Using Boron (III) Oxide and Neodymium (III) Oxide Substitution: Resistance Behaviors against Ionizing Radiation. Appl. Sci. 2021, 11, 3035. [Google Scholar] [CrossRef]
- Aygun, B. Neutron and gamma radiation shielding Ni based new type super alloys development and production by Monte Carlo Simulation technique. Radiat. Phys. Chem. 2021, 188, 109630. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Zhang, X.; Wu, H.; Guo, S.; Wang, Y. Enhancing the neutron shielding ability of polyethylene composites with an alternating multi-layered structure. Compos. Sci. Technol. 2021, 150, 16–23. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Sharkawy, R.M.; Allam, E.A.; Elsaman, R.; El-Taher, A. Fabrication and characterization of phosphotungstic acid-Copper oxide nanoparticles-Plastic waste nanocomposites for enhanced radiation-shielding. J. Alloy. Compd. 2019, 803, 768–777. [Google Scholar] [CrossRef]
- Al-Burahi, M.S.; Eke, C.; Alomairy, S.; Yildirim, A.; Alsaeedy, H.I.; Sriwunkum, C. Radiation attenuation properties of some commercial polymers for advanced shielding applications at low energies. Polym. Adv. Technol. 2021, 32, 2386–2396. [Google Scholar] [CrossRef]
- Mortazavi, S.; Kardan, M.; Sina, S.; Baharvand, H.; Sharafi, N. Design and fabrication of high density borated polyethylene nanocomposites as a neutron shield. Int. J. Radiat. Res. 2016, 14, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Olukotun, S.F.; Gbenu, S.T.; Oyedotun, K.O.; Fasakin, O.; Sayyed, M.I.; Akindoyin, G.O.; Shittu, H.O.; Fasasi, M.K.; Khandaker, M.U.; Osman, H.; et al. Fabrication and Characterization of Clay-Polyethylene Composite Opted for Shielding of Ionizing Radiation. Crystals 2021, 11, 1068. [Google Scholar] [CrossRef]
- Alabsy, M.T.; Alzahrani, J.S.; Sayyed, M.I.; Abbas, M.I.; Tishkevich, D.I.; El-Khatib, A.M.; Elsafi, M. Gamma-Ray Attenuation and Exposure Buildup Factor of Novel Polymers in Shielding Using Geant4 Simulation. Materials 2021, 14, 5051. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Almurayshid, M.; Alssalim, Y.; Aksouh, F.; Almsalam, R.; ALQahtani, M.; Sayyed, M.I.; Almasoud, F. Development of New Lead-Free Composite Materials as Potential Radiation Shields. Materials 2021, 14, 4957. [Google Scholar] [CrossRef]
- Muthamma, M.V.; Prabhu, S.; Bubbl, S.G.; Gudennavar, S.B. Micro and nano Bi2O3 filled epoxy composites: Thermal, mechanical and γ-ray attenuation properties. Appl. Radiat. Isot. 2021, 174, 109780. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, R.; Raszkowska-Kaczor, A.; Moraczewski, K.; Głuszewski, W.; Krasinskyi, V.; Wedderburn, L. The Structure and Mechanical Properties of Hemp Fibers-Reinforced Poly(ε-Caprolactone) Composites Modified by Electron Beam Irradiation. Appl. Sci. 2021, 11, 5317. [Google Scholar] [CrossRef]
- Güven, O. Radiation-Assisted Synthesis of Polymer-Based Nanomaterials. Appl. Sci. 2021, 11, 7913. [Google Scholar] [CrossRef]
- Acevedo-Del-Castillo, A.; Águila-Toledo, E.; Maldonado-Magnere, S.; Aguilar-Bolados, H. A Brief Review on the High-Energy Electromagnetic Radiation-Shielding Materials Based on Polymer Nanocomposites. Int. J. Mol. Sci. 2021, 22, 9079. [Google Scholar] [CrossRef]
- Doyan, A.; Susilawati, S.; Prayogi, S.; Bilad, M.R.; Arif, M.F.; Ismail, N.M. Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers 2021, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Smolyanskii, A.S.; Politova, E.D.; Koshkina, O.A.; Arsentyev, M.A.; Kusch, P.P.; Moskvitin, L.V.; Slesarenko, S.V.; Kiryukhin, D.P.; Trakhtenberg, L.I. Structure of Polytetrafluoroethylene Modified by the Combined Action of γ-Radiation and High Temperatures. Polymers 2021, 13, 3678. [Google Scholar] [CrossRef]
- Abu Saleem, R.A.; Abdelal, N.; Alsabbagh, A.; Al-Jarrah, M.; Al-Jawarneh, F. Radiation Shielding of Fiber Reinforced Polymer Composites Incorporating Lead Nanoparticles—An Empirical Approach. Polymers 2021, 13, 3699. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhuo, Y.; Li, L. SeO2 adsorption on CaO surface: DFT and experimental study on the adsorption of multiple SeO2 molecules. Appl. Surf. Sci. 2017, 420, 465–471. [Google Scholar] [CrossRef]
- López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol. 2007, 88, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.B.; Sloss, L.L. Trace Elements Emissions from Coal Combustion and Gasification. IEA Coal Res. 1992, IEACR/49. Available online: https://www.sustainable-carbon.org/report/trace-elements-emissions-from-coal-combustion-and-gasification-ieacr-49/ (accessed on 10 December 2021).
- Ahmad, M.S.; Yasser, M.M.; Sholkamy, E.N.; Ali, A.M.; Mehanni, M.M. Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int. J. Nanomed. 2015, 10, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Pan, X.; Gadd, G.M. Soil dissolved organic matter affects mercury immobilization by biogenicselenium nanoparticles. Sci. Total Environ. 2019, 658, 8–15. [Google Scholar] [CrossRef]
- Elshami, W.; Tekin, H.O.; Al-Buriahi, M.S.; Hegazy, H.H.; Abuzaid, M.M.; Issa, S.A.M.; Zaid, M.H.M.; Sidek, H.A.A.; Matori, K.A.; Zakaly, H.M.H. Developed selenium dioxide-based ceramics for advanced shielding applications: Au2O3 impact on nuclear radiation attenuation. Results Phys. 2021, 24, 104099. [Google Scholar] [CrossRef]
- Kebaili, I.; Boukhris, I.; Al-Buriahi, M.S.; Alalawi, A.; Sayyed, M.I. Ge-Se-Sb-Ag chalcogenide glasses for nuclear radiation shielding applications. Ceram. Int. 2021, 47, 1303–1309. [Google Scholar] [CrossRef]
- El-Qahtani, Z.M.H.; Shaaban, E.R.; Soraya, M.M. Attenuation characteristics of high-energy radiation on As-Se-Sn chalcogenide glassy alloy. Chalcogenide Lett. 2021, 18, 311–326. [Google Scholar]
- Kebaili, I.; Znaidia, S.; Alzahrani, J.S.; Alothman, M.A.; Boukhris, I.; Olarinoye, I.O.; Mutuwong, C.; Al-Buriahi, M.S. Ge20Se80−xBix (x ≤ 12) chalcogenide glasses for infrared and gamma sensing applications: Structural, optical and gamma attenuation aspects. J. Mater. Sci. Mater. Electron. 2021, 32, 15509–15522. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I. Gamma Ray Shielding Properties of Yb3+-Doped Calcium Borotellurite Glasses. Appl. Sci. 2021, 11, 5697. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I.; Albarzan, B.; Javier-Hila, A.M.V.; Alwadai, N.; Kumar, A. Mechanical and Gamma-Ray Interaction Studies of PbO–MoO3–Li2O–B2O3 Glass System for Shielding Applications in The Low Energy Region: A Theoretical Approach. Appl. Sci. 2021, 11, 5538. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Hamad, M.K.; Abu Mhareb, M.H.; Naseer, K.A.; Mahmoud, K.A.; Khandaker, M.U.; Osman, H.; Elesawy, B.H. Impact of Modifier Oxides on Mechanical and Radiation Shielding Properties of B2O3-SrO-TeO2-RO Glasses (Where RO = TiO2, ZnO, BaO, and PbO). Appl. Sci. 2021, 11, 10904. [Google Scholar] [CrossRef]
- Albarzan, B.; Hanfi, M.Y.; Almuqrin, A.H.; Sayyed, M.I.; Alsafi, H.M.; Mahmoud, K.A. The Influence of Titanium Dioxide on Silicate-Based Glasses: An Evaluation of the Mechanical and Radiation Shielding Properties. Materials 2021, 14, 3414. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Dong, M.; Sayyed, M.I.; Wang, C.; Almuqrin, A.H.; Almasoud, F.I. The Vital Role of La2O3 on the La2O3-CaO-B2O3-SiO2 Glass System for Shielding Some Common Gamma Ray Radioactive Sources. Materials 2021, 14, 4776. [Google Scholar] [CrossRef]
- El-Nahal, M.A.; Elsafi, M.; Sayyed, M.I.; Khandaker, M.U.; Osman, H.; Elesawy, B.H.; Saleh, I.H.; Abbas, M.I. Understanding the Effect of Introducing Micro- and Nanoparticle Bismuth Oxide (Bi2O3) on the Gamma Ray Shielding Performance of Novel Concrete. Materials 2021, 14, 6487. [Google Scholar] [CrossRef] [PubMed]
- Elsafi, M.; Dib, M.F.; Mustafa, H.E.; Sayyed, M.I.; Khandaker, M.U.; Alsubaie, A.; Almalki, A.S.A.; Abbas, M.I.; El-Khatib, A.M. Enhancement of Ceramics Based Red-Clay by Bulk and Nano Metal Oxides for Photon Shielding Features. Materials 2021, 14, 7878. [Google Scholar] [CrossRef]
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.; Zucker, D.S.; Olsen, K. XCOM: Photon Cross Sections Database-NIST Standard Reference Database 8 (XGAM). NIST PML Radiat. Phys. Div. NBSIR 2010, 87–3597. Available online: https://doi.org/10.18434/T48G6X (accessed on 10 December 2021).
- Gerward, L.; Guilbert, N.; Jensen, K.B.; Levring, H. X-ray absorption in matter. Reengineering XCOM. Radiat. Phys. Chem. 2001, 60, 23–24. [Google Scholar] [CrossRef]
- Gerward, L.; Guilbert, N.K.; Jensen, B.; Levring, H. WinXCom—A program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 2004, 71, 653–654. [Google Scholar] [CrossRef]
- Kavaz, E.; Ekinci, N.; Tekin, H.O.; Sayyed, M.I.; Aygun, B.; Perisanoglu, U. Estimation of gamma radiation shielding qualification of newly developed glasses by using WinXCOM and MCNPX code. Prog. Nucl. Energy 2019, 115, 12–20. [Google Scholar] [CrossRef]
- Savaskan, S. Synthesis and Investigation of Ion Exchange Properties of New Ion Exchangers. Ph.D. Thesis, Graduate School of Natural and Applied Sciences Institute, Chemistry Department, KTU, Trabzon, Turkey, March 1994. [Google Scholar]
- Savaşkan, S.; Besşïrlï, N.; Hazer, B. Synthesis of some new cation-exchanger resins. J. Appl. Polym. Sci. 1996, 59, 1515–1524. [Google Scholar] [CrossRef]
- Savaskan Yilmaz, S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E. Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation. Materials 2020, 13, 4390. [Google Scholar] [CrossRef]
- Savaskan Yilmaz, S.; Kul, D.; Erdöl, M.; Özdemir, M.; Abbasoğlu, R. Synthesis of a novel crosslinked superabsorbent copolymer with diazacyclooctadecane crown ether and its sorption capability. Eur. Polym. J. 2007, 43, 1923–1932. [Google Scholar] [CrossRef]





















| Line | Nanocomposite Label | PS-b-PEG Type | PS-b-PEG (wt%) | BN (wt%) | SeO2 (wt%) |
|---|---|---|---|---|---|
| 1 | PSNC1 | 1000 | 100 | 0 | 0 |
| 2 | PSNC2 | 1000 | 50 | 0 | 50 |
| 3 | PSNC3 | 1000 | 30 | 0 | 70 |
| 4 | PSNC4 | 1000 | 10 | 0 | 90 |
| 5 | PSNC5 | 1000 | 46.2 | 0 | 53.8 |
| 6 | PSNC6 | 1500 | 100 | 0 | 0 |
| 7 | PSNC7 | 1500 | 50 | 0 | 50 |
| 8 | PSNC8 | 1500 | 30 | 0 | 70 |
| 9 | PSNC9 | 1500 | 10 | 0 | 90 |
| 10 | PSNC10 | 1500 | 46.2 | 0 | 53.8 |
| 11 | PSNC11 | 10,000 | 100 | 0 | 0 |
| 12 | PSNC12 | 10,000 | 50 | 0 | 50 |
| 13 | PSNC13 | 10,000 | 30 | 0 | 70 |
| 14 | PSNC14 | 10,000 | 10 | 0 | 90 |
| 15 | PSNC15 | 10,000 | 46.2 | 0 | 53.8 |
| 16 | PBSNC1 | 1000 | 50 | 50 | 0 |
| 17 | PBSNC2 | 1000 | 15 | 15 | 70 |
| 18 | PBSNC3 | 1000 | 5 | 5 | 90 |
| 19 | PBSNC4 | 1000 | 26.1 | 13 | 60.9 |
| 20 | PBSNC5 | 1500 | 50 | 50 | 0 |
| 21 | PBSNC6 | 1500 | 15 | 15 | 70 |
| 22 | PBSNC7 | 1500 | 5 | 5 | 90 |
| 23 | PBSNC8 | 1500 | 26.1 | 13 | 60.9 |
| 24 | PBSNC9 | 10,000 | 50 | 50 | 0 |
| 25 | PBSNC10 | 10,000 | 15 | 15 | 70 |
| 26 | PBSNC11 | 10,000 | 5 | 5 | 90 |
| 27 | PBSNC12 | 10,000 | 26.1 | 13 | 60.9 |
| Radiation Shielding Factors | Units | Equations | References |
|---|---|---|---|
| ) | cm2/g | [11,12] | |
| ) | cm−1 | [11,12] | |
| Radiation protection efficiency (RPE) | - | [11,12] | |
| Half-value layer (HVL) | cm | [11,12] | |
| Tenth-value layer (TVL) | cm | [11,12] | |
| Mean free path (MFP) | cm | [11,12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinan, Z.M.; Erol, B.; Baskan, T.; Mutlu, S.; Ortaç, B.; Savaskan Yilmaz, S.; Yilmaz, A.H. Radiation Shielding Tests of Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers Blended with Nanostructured Selenium Dioxide and Boron Nitride Particles. Nanomaterials 2022, 12, 297. https://doi.org/10.3390/nano12030297
Cinan ZM, Erol B, Baskan T, Mutlu S, Ortaç B, Savaskan Yilmaz S, Yilmaz AH. Radiation Shielding Tests of Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers Blended with Nanostructured Selenium Dioxide and Boron Nitride Particles. Nanomaterials. 2022; 12(3):297. https://doi.org/10.3390/nano12030297
Chicago/Turabian StyleCinan, Zehra Merve, Burcu Erol, Taylan Baskan, Saliha Mutlu, Bülend Ortaç, Sevil Savaskan Yilmaz, and Ahmet Hakan Yilmaz. 2022. "Radiation Shielding Tests of Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers Blended with Nanostructured Selenium Dioxide and Boron Nitride Particles" Nanomaterials 12, no. 3: 297. https://doi.org/10.3390/nano12030297