Insight into the Properties of Plasmonic Au/TiO2 Activated by O2/Ar Plasma
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Au/TiO2 Photocatalysts
2.2. Plasma Activation and Measurement of Electric Discharge Characteristics
2.3. Photocatalytic Evaluation
2.4. Photocatalyst Characterization
3. Results and Discussion
3.1. Electrical Discharge Characteristics
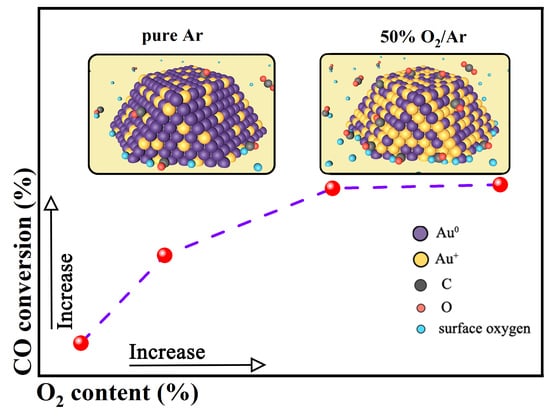
3.2. Photocatalytic Performance
3.3. Optical Property
3.4. TEM Observation
3.5. Surface Chemical State Analysis
3.6. CO Chemisorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Liang, S.; Gu, Q.; Xie, L.; Wang, J.; Ding, Z.; Liu, P. Effect of Au supported TiO2 with dominant exposed {001} facets on the visible-light photocatalytic activity. Appl. Catal. B-Environ. 2012, 119–120, 146–155. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, Y.; Lee, S.-T.; Yang, S.; Kang, Z. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon Resonant Enhancement of Photocatalytic Water Splitting Under Visible Illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Wang, X.; Long, R.; Liu, D.; Yang, D.; Wang, C.; Xiong, Y. Enhanced full-spectrum water splitting by confining plasmonic Au nanoparticles in N-doped TiO2 bowl nanoarrays. Nano Energy 2016, 24, 87–93. [Google Scholar] [CrossRef]
- Naldoni, A.; Riboni, F.; Marelli, M.; Bossola, F.; Ulisse, G.; Di Carlo, A.; Píš, I.; Nappini, S.; Malvestuto, M.; Dozzi, M.V.; et al. Influence of TiO2electronic structure and strong metal–support interaction on plasmonic Au photocatalytic oxidations. Catal. Sci. Technol. 2016, 6, 3220–3229. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Corti, C.W.; Holliday, R.J.; Thompson, D.T. Commercial aspects of gold catalysis. Appl. Catal. A-Gen. 2005, 291, 253–261. [Google Scholar] [CrossRef]
- Li, W.; Comotti, M.; Schüth, F. Highly reproducible syntheses of active Au/TiO2 catalysts for CO oxidation by deposition–precipitation or impregnation. J. Catal. 2006, 237, 190–196. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Zhao, J.; Zheng, Z.; Gao, X. Visible-Light-Driven Oxidation of Organic Contaminants in Air with Gold Nanoparticle Catalysts on Oxide Supports. Angew. Chem. Int. Ed. 2008, 47, 5353–5356. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Si, R.; Zheng, H.; Geng, Q.; Dai, W.; Chen, X.; Fu, X. The promoted oxidation of CO induced by the visible-light response of Au nanoparticles over Au/TiO2. Catal. Commun. 2012, 26, 136–139. [Google Scholar] [CrossRef]
- Haruta, M. Chance and Necessity: My Encounter with Gold Catalysts. Angew. Chem. Int. Ed. 2014, 53, 52–56. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of Recent Advances of Dielectric Barrier Discharge Plasma in Catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 034005. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced Photocatalytic Degradation of Organic Dyes via Defect-Rich TiO2 Prepared by Dielectric Barrier Discharge Plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Bu, D.; Li, Z.; Zhang, X.; Di, L. Cold Plasma Preparation of Pd/Graphene Catalyst for Reduction of p-Nitrophenol. Nanomaterials 2021, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rimmer, R.; Gunamgari, J.; Shekhawat, R.; Davis, B.; Mazumder, M.; Lindquist, D. Plasma-Assisted Activation of Supported Au and Pd Catalysts for CO Oxidation. IEEE Trans. Ind. Appl. 2005, 41, 1373–1376. [Google Scholar] [CrossRef]
- Di, L.; Zhan, Z.; Zhang, X.; Qi, B.; Xu, W.; Lanbo, D.; Zhibin, Z.; Xiuling, Z.; Bin, Q.; Weijie, X. Atmospheric-Pressure DBD Cold Plasma for Preparation of High Active Au/P25 Catalysts for Low-Temperature CO Oxidation. Plasma Sci. Technol. 2016, 18, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Di, L.; Yu, F.; Duan, D.; Zhang, X. Atmospheric-Pressure Cold Plasma Activating Au/P25 for CO Oxidation: Effect of Working Gas. Nanomaterials 2018, 8, 742. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mou, C.-Y.; Lee, S.; Li, Y.; Secrest, J.; Jang, B.W.-L. Room temperature O2 plasma treatment of SiO2 supported Au catalysts for selective hydrogenation of acetylene in the presence of large excess of ethylene. J. Catal. 2012, 285, 152–159. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.-S.; Zhu, B.; Liu, J.-L.; Zhu, X.; Zhu, A.-M.; Jang, B.W.-L. Atmospheric-pressure O2 plasma treatment of Au/TiO2 catalysts for CO oxidation. Catal. Today 2015, 256, 142–147. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, X.-S.; Liu, J.-L.; Li, Y.-C.; Zhu, B.; Zhu, A.-M. A promising visible-light photocatalyst: H2 plasma-activated amorphous-TiO2-supported Au nanoparticles. J. Catal. 2019, 375, 380–388. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xie, Y.; Liu, C.-J. Synthesis and Characterization of Noble Metal (Pd, Pt, Au, Ag) Nanostructured Materials Confined in the Channels of Mesoporous SBA-15. J. Phys. Chem. C 2008, 112, 19818–19824. [Google Scholar] [CrossRef]
- Liu, C.-J.; Zhao, Y.; Li, Y.; Zhang, D.-S.; Chang, Z.; Bu, X.-H. Perspectives on Electron-Assisted Reduction for Preparation of Highly Dispersed Noble Metal Catalysts. ACS Sustain. Chem. Eng. 2014, 2, 3–13. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Zhu, B.; Li, X.-S.; Liu, J.-L.; Zhu, X.; Zhu, A.-M. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B-Environ. 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Liu, J.-L.; Li, X.-S.; Zhu, B.; Zhu, X.; Zhu, A.-M. Kinetic study on visible-light photocatalytic removal of formaldehyde from air over plasmonic Au/TiO2. Catal. Today 2017, 281, 630–635. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.-Y.; Liu, J.-L.; Zhang, X.-M.; Li, X.-S.; Zhu, A.-M. TiO2-supported Au-Ag plasmonic nanocatalysts achieved by plasma restructuring and activation. J. Hazard. Mater. 2021, 402, 123508. [Google Scholar] [CrossRef]
- Kogelschatz, U. Filamentary, patterned, and diffuse barrier discharges. IEEE Trans. Plasma Sci. 2002, 30, 1400–1408. [Google Scholar] [CrossRef]
- Rahel, J.; Sherman, D.M. The transition from a filamentary dielectric barrier discharge to a diffuse barrier discharge in air at atmospheric pressure. J. Phys. D Appl. Phys. 2005, 38, 547–554. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Liu, J.-L.; Liu, J.-B.; Zhu, X.; Zhu, A.-M. In-situ regeneration of Au nanocatalysts by atmospheric-pressure air plasma: Significant contribution of water vapor. Appl. Catal. B-Environ. 2015, 179, 69–77. [Google Scholar] [CrossRef]
- Masoud, N.; Martus, K.; Figus, M.; Becker, K. Rotational and Vibrational Temperature Measurements in a High-Pressure Cylindrical Dielectric Barrier Discharge (C-DBD). Contrib. Plasma Phys. 2005, 45, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Li, Y.; Xiao, H.; Li, Y.; Tang, J. Study on Degradation of SF6 in the Presence of H2O and O2 Using Dielectric Barrier Discharge. IEEE Access 2018, 6, 72748–72756. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Sau, T.K.; Murphy, C.J. Shape-Dependent Plasmon-Resonant Gold Nanoparticles. Small 2006, 2, 636–639. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 1. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Shin, C.H.; Henry, C.R.; Louis, C. Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J. Catal. 2004, 222, 357–367. [Google Scholar] [CrossRef]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition–precipitation with NaOH and urea. Appl. Catal. A-Gen. 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Zanella, R.; Louis, C. Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples. Catal. Today 2005, 107–108, 768–777. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Manzolia, M.; Lub, P.; Akitab, T.; Ichikawab, S.; Harutab, M. Au/TiO2 Nanosized Samples: A Catalytic, TEM, and FTIR Study of the Effect of Calcination Temperature on the CO Oxidation. J. Catal. 2001, 202, 256–267. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Groult, H.; Marcus, P. Surface study of a titanium-based ceramic electrode material by X-ray photoelectron spectroscopy. J. Mater. Sci. 1997, 32, 5645–5651. [Google Scholar] [CrossRef]
- Biener, J.; Farfan-Arribas, E.; Biener, M.; Friend, C.M.; Madix, R.J. Synthesis of TiO2 nanoparticles on the Au(111) surface. J. Chem. Phys. 2005, 123, 094705. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Quinteros-Jara, E.; Cuenca-Bracamonte, Q.; Quijada, R.; Carretero-González, J.; Avilés, F.; Lopez-Manchado, M.A.; Verdejo, R. Synthesis of sustainable, lightweight and electrically conductive polymer brushes grafted multi-layer graphene oxide. Polym. Test. 2021, 93, 106986. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Zhao, Q. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method. Thin Solid Film. 2000, 379, 7–14. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.W.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B-Environ. 2008, 84, 440–447. [Google Scholar] [CrossRef]
- Dementjev, A.; de Graaf, A.; van de Sanden, M.; Maslakov, K.; Naumkin, A.; Serov, A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Fierro-Gonzalez, J.; Gates, B.C. Evidence of active species in CO oxidation catalyzed by highly dispersed supported gold. Catal. Today 2007, 122, 201–210. [Google Scholar] [CrossRef]
- Bollinger, M.A.; Vannice, M.A. A kinetic and DRIFTS study of low-temperature carbon monoxide oxidation over Au—TiO2 catalysts. Appl. Catal. B-Environ. 1996, 8, 417–443. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A. FTIR Study of CO Oxidation on Au/TiO2 at 90 K and Room Temperature. An Insight into the Nature of the Reaction Centers. J. Phys. Chem. B 2000, 104, 5414–5416. [Google Scholar] [CrossRef]
- Molina, L.M.; Rasmussen, M.D.; Hammer, B. Adsorption of O2 and oxidation of CO at Au nanoparticles supported by TiO2(110). J. Chem. Phys. 2004, 120, 7673–7680. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P.; Corma, A. Unravelling the Nature of Gold Surface Sites by Combining IR Spectroscopy and DFT Calculations. Implications in Catalysis. J. Phys. Chem. C 2009, 113, 16772–16784. [Google Scholar] [CrossRef]
- Gaur, S.; Wu, H.; Stanley, G.G.; More, K.; Kumar, C.S.; Spivey, J.J. CO oxidation studies over cluster-derived Au/TiO2 and AUROlite™ Au/TiO2 catalysts using DRIFTS. Catal. Today 2013, 208, 72–81. [Google Scholar] [CrossRef]
- Hao, Y.; Mihaylov, M.; Ivanova, E.; Hadjiivanov, K.; Knözinger, H.; Gates, B. CO oxidation catalyzed by gold supported on MgO: Spectroscopic identification of carbonate-like species bonded to gold during catalyst deactivation. J. Catal. 2009, 261, 137–149. [Google Scholar] [CrossRef]








| Samples | Proportion (at.%) | ||||
|---|---|---|---|---|---|
| Osurf (~531.6 eV)/O | Au0/Au | Au+/Au | C-O/C | C-OO/C | |
| Ar | 14.4 | 68.0 | 32.0 | 32.0 | |
| 10% O2/Ar | 16.1 | 67.4 | 32.6 | 8.6 | 5.3 |
| 30% O2/Ar | 19.6 | 63.3 | 36.7 | 11.9 | 5.9 |
| 50% O2/Ar | 20.0 | 60.2 | 39.8 | 16.3 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Ding, Y.; Wang, X.; Jia, X.; Zhang, S.; Li, X. Insight into the Properties of Plasmonic Au/TiO2 Activated by O2/Ar Plasma. Nanomaterials 2022, 12, 106. https://doi.org/10.3390/nano12010106
Deng X, Ding Y, Wang X, Jia X, Zhang S, Li X. Insight into the Properties of Plasmonic Au/TiO2 Activated by O2/Ar Plasma. Nanomaterials. 2022; 12(1):106. https://doi.org/10.3390/nano12010106
Chicago/Turabian StyleDeng, Xiaoqing, Yu Ding, Xiaobing Wang, Xiaojing Jia, Shuo Zhang, and Xiang Li. 2022. "Insight into the Properties of Plasmonic Au/TiO2 Activated by O2/Ar Plasma" Nanomaterials 12, no. 1: 106. https://doi.org/10.3390/nano12010106