Incorporation of Biomass-Based Carbon Nanoparticles into Polysulfone Ultrafiltration Membranes for Enhanced Separation and Anti-Fouling Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BCNPs
2.3. Fabrication of PSF/BCNPs Composite Membranes
2.4. Characterizations of BCNPs
2.5. Characterizations of the Membranes
2.6. Performances of the Membranes
3. Results and Discussion
3.1. Characterizations of BCNPs
3.2. Characterizations of PSF/BCNPs Composite Membranes
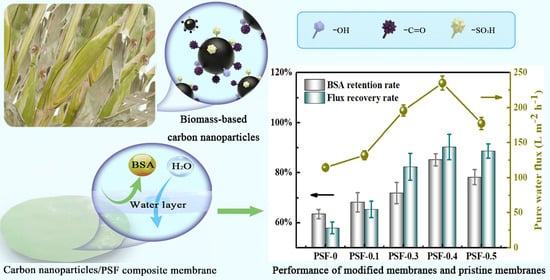
3.3. Performance of PSF/BCNPs Composite Membranes
| Modified Materials | % Increase in BSA Retention | FRR | References |
|---|---|---|---|
| Biomass-based carbon nanoparticles | 21.7 | 90.3% | This work |
| Guanidyl-functionalized graphene | 0.5 | 82.4% | [28] |
| Glucose-based carbon nanospheres | ~9.0 | ~83.0% | [9] |
| Sulfonated graphene oxide | nearly unchanged | – | [6] |
| Oxidized carbon nanotubes | 21.7 | 72.8% | [2] |
| Graphene oxide | 17.2 | 85.1% | [2] |
| Oxidized carbon nanotubes and graphene oxide | 14.2 | 80.4% | [2] |
| Graphene oxide | ~2.0 | 69.0% | [29] |
| Graphene oxide-polyethylene glycol | ~−2 | 78.0% | [29] |
| Cysteine-functionalized graphene oxide | ~3.0 | 92.1% | [19] |
| PDA-functionalized GO | nearly unchanged | 86.9% | [30] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Zhang, J.G.; Xu, Z.W.; Mai, W.; Min, C.Y.; Zhou, B.M.; Shan, M.J.; Li, Y.L.; Yang, C.Y.; Wang, Z.; Qian, X.M. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Selakjani, P.P.; Peyravi, M.; Jahanshahi, M.; Hoseinpour, H.; Rad, A.S.; Khalili, S. Strengthening of polysulfone membranes using hybrid mixtures of micro- and nano-scale modifiers. Front. Chem. Sci. Eng. 2018, 12, 174–183. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.G.; Pan, X.J.; Zhang, L.; Chen, H.L.; Gao, C.J. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Koulivand, H.; Shahbazi, A.; Vatanpour, V.; Rahmandoost, M. Novel antifouling and antibacterial polyethersulfone membrane prepared by embedding nitrogen-doped carbon dots for efficient salt and dye rejection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110787. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Yu, H.; Gu, L.; Wu, S.; Dong, G.; Qiao, X.; Zhang, K.; Lu, X.; Wen, H.; Zhang, D. Hydrothermal carbon nanospheres assisted-fabrication of PVDF ultrafiltration membranes with improved hydrophilicity and antifouling performance. Sep. Purif. Technol. 2020, 247, 116889. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Gontarek-Castro, E.; Ollik, K.; Lieder, M. Biomass-derived nitrogen functionalized carbon nanodots and their anti-biofouling properties. Processes 2021, 9, 61. [Google Scholar] [CrossRef]
- Mofradi, M.; Karimi, H.; Dashtian, K.; Ghaedi, M. Corn derivative mesoporous carbon microspheres supported hydrophilic polydopamine for development of new membrane: Water treatment containing bovine serum albumin. Chemosphere 2020, 259, 127440. [Google Scholar] [CrossRef]
- Mahat, N.A.; Shamsudin, S.A.; Jullok, N.; Ma’Radzi, A.H. Carbon quantum dots embedded polysulfone membranes for antibacterial performance in the process of forward osmosis. Desalination 2020, 493, 114618. [Google Scholar] [CrossRef]
- Liu, X.D.; Yuan, H.K.; Wang, C.C.; Zhang, S.; Zhang, L.J.; Liu, X.J.; Liu, F.J.; Zhu, X.Y.; Rohani, S.; Ching, C.B.; et al. A novel PVDF/PFSA-g-GO ultrafiltration membrane with enhanced permeation and antifouling performances. Sep. Purif. Technol. 2020, 233, 116038. [Google Scholar] [CrossRef]
- Abdollahi, E.; Heidari, A.; Mohammadi, T.; Asadi, A.A.; Tofighy, M.A. Application of Mg-Al LDH nanoparticles to enhance flux, hydrophilicity and antifouling properties of PVDF ultrafiltration membrane: Experimental and modeling studies. Sep. Purif. Technol. 2021, 257, 117931. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.L.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.G.; Kang, H.; Han, S.; Lee, J.C. The increase of antifouling properties of ultrafiltration membrane coated by star-shaped polymers. J. Mater. Chem. 2012, 22, 8654–8661. [Google Scholar] [CrossRef]
- Ma, S.; Jing, F.; Sohi, S.P.; Chen, J. New insights into contrasting mechanisms for PAE adsorption on millimeter, micron- and nano-scale biochar. Environ. Sci. Pollut. Res. 2019, 26, 18636–18650. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.N.; Zheng, Z.Y.; Liu, Y.L.; Jiang, X.Z.; Wu, J.M.; Feng, M.; Xu, L.; Guan, Q.B.; Li, H.T. Photo-responsive shape memory polymer composites enabled by doping with biomass-derived carbon nanomaterials. Nano Res. 2021, 1–10. [Google Scholar]
- Kong, S.; Lim, M.Y.; Shin, H.; Baik, J.H.; Lee, J.C. High-flux and antifouling polyethersulfone nanocomposite membranes incorporated with zwitterion-functionalized graphene oxide for ultrafiltration applications. J. Ind. Eng. Chem. 2020, 84, 131–140. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Zebarjad, S.M.; Janghorban, K. Decoration of multi-walled carbon nanotubes with silver nanoparticles and investigation on its colloid stability. Mater. Chem. Phys. 2013, 139, 113–117. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Lavanya, C.; Balakrishna, R.G. Naturally derived polysaccharides-modified PSF membranes: A potency in enriching the antifouling nature of membranes. Sep. Purif. Technol. 2020, 230, 115887. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wei, X.; Zhao, B.R.; Wang, J.X.; Yang, S.B.; Wang, S.C. Performance improvement of polysulfone ultrafiltration membrane using PANiEB as both pore forming agent and hydrophilic modifier. J. Membr. Sci. 2011, 385, 251–262. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zhou, S.X.; Yang, H.H.; Gu, G.X.; Wu, L.M. Preparation and characterization of nanocomposite polyurethane. J. Colloid Interface Sci. 2004, 279, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Zain, N.M.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Zhang, G.L.; Zhou, M.; Xu, Z.H.; Jiang, C.Y.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 295–305. [Google Scholar] [CrossRef]
- Ma, C.; Hu, J.; Sun, W.J.; Ma, Z.G.; Yang, W.Q.; Wang, L.; Ran, Z.L.; Zhao, B.; Zhang, Z.H.; Zhang, H.W. Graphene oxide-polyethylene glycol incorporated PVDF nanocomposite ultrafiltration membrane with enhanced hydrophilicity, permeability, and antifouling performance. Chemosphere 2020, 253, 126649. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Membr. Sci. 2021, 620, 118900. [Google Scholar] [CrossRef]






| PSF (wt%) | BCNPs-DMF (wt%) | DMF (wt%) | Total (wt%) | |
|---|---|---|---|---|
| PSF-0 | 17.0 | 0.0 | 83.0 | 100.0 |
| PSF-0.1 | 17.0 | 17.0 | 66.0 | 100.0 |
| PSF-0.3 | 17.0 | 51.0 | 32.0 | 100.0 |
| PSF-0.4 | 17.0 | 68.0 | 15.0 | 100.0 |
| PSF-0.5 | 17.0 | 83.0 | 0.0 | 100.0 |
| Atomic Ratio (wt%) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|
| C | O | N | S | ||
| CSs | 64.0 | 33.7 | 2.3 | 0.0 | / |
| BCNPs | 52.1 | 41.4 | 2.0 | 4.5 | −42.9 |
| Membrane | Thickness (μm) | Porosity (%) | Mean Pore Size (nm) | Surface Roughness Parameters (nm) | |||
|---|---|---|---|---|---|---|---|
| a 1 | b 2 | Ra | Rq | Rz | |||
| PSF-0 | 48.3 | 70.3 | 30.1 | 28.2 | 7.3 | 5.6 | 53.4 |
| PSF-0.1 | 48.6 | 72.0 | 31.7 | 32.5 | 7.0 | 5.5 | 51.0 |
| PSF-0.3 | 48.8 | 72.7 | 38.2 | 42.3 | 6.4 | 5.0 | 46.9 |
| PSF-0.4 | 49.2 | 73.7 | 41.4 | 48.3 | 5.6 | 4.5 | 34.8 |
| PSF-0.5 | 49.0 | 72.7 | 36.4 | 46.2 | 6.2 | 5.0 | 39.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Chen, J.; Wu, J.; Feng, M.; Xu, L.; Yan, N.; Xie, H. Incorporation of Biomass-Based Carbon Nanoparticles into Polysulfone Ultrafiltration Membranes for Enhanced Separation and Anti-Fouling Performance. Nanomaterials 2021, 11, 2303. https://doi.org/10.3390/nano11092303
Zheng Z, Chen J, Wu J, Feng M, Xu L, Yan N, Xie H. Incorporation of Biomass-Based Carbon Nanoparticles into Polysulfone Ultrafiltration Membranes for Enhanced Separation and Anti-Fouling Performance. Nanomaterials. 2021; 11(9):2303. https://doi.org/10.3390/nano11092303
Chicago/Turabian StyleZheng, Zhiyu, Jingwen Chen, Jiamin Wu, Min Feng, Lei Xu, Nina Yan, and Hongde Xie. 2021. "Incorporation of Biomass-Based Carbon Nanoparticles into Polysulfone Ultrafiltration Membranes for Enhanced Separation and Anti-Fouling Performance" Nanomaterials 11, no. 9: 2303. https://doi.org/10.3390/nano11092303