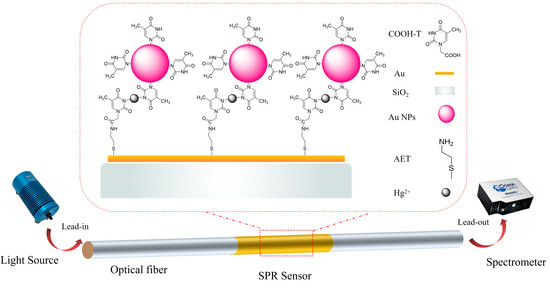
Thymine-Functionalized Gold Nanoparticles (Au NPs) for a Highly Sensitive Fiber-Optic Surface Plasmon Resonance Mercury Ion Nanosensor
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stern, A.H. A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment. Environ. Res. 2005, 98, 133–142. [Google Scholar] [CrossRef]
- Uzun, L.; Kara, A.; Osman, B.; Yılmaz, E.; Beşirli, N.; Denizli, A. Removal of heavy-metal ions by magnetic beads containing triazole chelating groups. J. Appl. Polym. Sci. 2009, 114, 2246–2253. [Google Scholar] [CrossRef]
- Dai, X.; Nekrassova, O.; Hyde, A.M.E.; Compton, R.G. Anodic Stripping Voltammetry of Arsenic(III) Using Gold Nanoparticle-Modified Electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Cho, M.; Choe, W.-S.; Son, Y.; Lee, Y. Electrochemical detection of copper ion using a modified copolythiophene electrode. Electrochim. Acta 2009, 54, 7012–7017. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. (In English) [Google Scholar] [CrossRef] [Green Version]
- Laffont, L.; Hezard, T.; Gros, P.; Heimbürger, L.-E.; Sonke, J.E.; Behra, P.; Evrard, D. Mercury(II) trace detection by a gold nanoparticle-modified glassy carbon electrode using square-wave anodic stripping voltammetry including a chloride desorption step. Talanta 2015, 141, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Costas-Mora, I.; Romero, V.; Lavilla, I.; Bendicho, C. In Situ Building of a Nanoprobe Based on Fluorescent Carbon Dots for Methylmercury Detection. Anal. Chem. 2014, 86, 4536–4543. [Google Scholar] [CrossRef]
- Holmes, P.; James, K.; Levy, L. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. (In English) [Google Scholar] [CrossRef]
- Kobal, A.B.; Horvat, M.; Prezelj, M.; Briški, A.S.; Krsnik, M.; Dizdarevič, T.; Mazej, D.; Falnoga, I.; Stibilj, V.; Arnerič, N.; et al. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J. Trace Elements Med. Biol. 2004, 17, 261–274. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Anagnostopoulos, V.A.; Manariotis, I.D.; Karapanagioti, H.K.; Chrysikopoulos, C.V. Removal of mercury from aqueous solutions by malt spent rootlets. Chem. Eng. J. 2012, 213, 135–141. [Google Scholar] [CrossRef]
- Guha, S.; Lohar, S.; Hauli, I.; Mukhopadhyay, S.K.; Das, D. Vanillin-coumarin hybrid molecule as an efficient fluorescent probe for trace level determination of Hg(II) and its application in cell imaging. Talanta 2011, 85, 1658–1664. [Google Scholar] [CrossRef]
- Li, D.; Wieckowska, A.; Willner, I. Optical Analysis of Hg2+ Ions by Oligonucleotide–Gold-Nanoparticle Hybrids and DNA-Based Machines. Angew. Chem. Int. Ed. Engl. 2008, 120, 3991–3995. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Zhang, J.; Wang, C.; Li, L.; Man, Q.; Lundebye, A.-K.; Frøyland, L. Risk–benefit evaluation of fish from Chinese markets: Nutrients and contaminants in 24 fish species from five big cities and related assessment for human health. Sci. Total Environ. 2012, 416, 187–199. [Google Scholar] [CrossRef]
- Yuan, X.; Li, K.; Zhang, Y.; Miao, Y.; Xiang, Y.; Sha, Y.; Zhang, M.; Huang, K. Point discharge microplasma for the determination of mercury in Traditional Chinese Medicines by chemical vapor generation atomic emission spectrometry. Microchem. J. 2020, 155, 104695. [Google Scholar] [CrossRef]
- He, C.; Cheng, G.; Zheng, C.; Wu, L.; Lee, Y.-I.; Hou, X. Photochemical vapor generation and in situ preconcentration for determination of mercury by graphite furnace atomic absorption spectrometry. Anal. Methods 2015, 7, 3015–3021. [Google Scholar] [CrossRef]
- Shi, M.-T.; Yang, X.-A.; Zhang, W.-B. Magnetic graphitic carbon nitride nano-composite for ultrasound-assisted dispersive micro-solid-phase extraction of Hg(II) prior to quantitation by atomic fluorescence spectroscopy. Anal. Chim. Acta 2019, 1074, 33–42. [Google Scholar] [CrossRef]
- Zeng, R.J.; Zhang, L.J.; Su, L.S.; Luo, Z.B.; Zhou, Q.; Tang, D.P. Photoelectrochemical bioanalysis of antibiotics on rGO-Bi2WO6-Au based on branched hybridization chain reaction. Biosens. Bioelectron. 2019, 133, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Umesh, S.; Murali, S.; Asokan, S.; Gorthi, S.S. Bare fiber Bragg grating immunosensor for real-time detection of Escherichia coli bacteria. J. Biophoton. 2017, 10, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Li, L.; Zhang, Y.; Wang, X.; Ozaki, Y. Recent advances in surface-enhanced Raman scattering-based sensors for the detection of inorganic ions: Sensing mechanism and beyond. J. Raman Spectrosc. 2020. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.-Y.; Wang, B.-T. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Lu, X.; Liu, Q.; Wang, F.; Lv, J.; Sun, T.; Mu, H.; Chu, P.K. Mid-infrared surface plasmon resonance sensor based on photonic crystal fibers. Opt. Express 2017, 25, 14227–14237. [Google Scholar] [CrossRef] [PubMed]
- Monfared, Y.E. Refractive Index Sensor Based on Surface Plasmon Resonance Excitation in a D-Shaped Photonic Crystal Fiber Coated by Titanium Nitride. Plasmonics 2019, 15, 535–542. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.; Sudarsanakumar, C. Surface Plasmon Resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar]
- Chen, Z.; Han, K.; Zhang, Y.-N. Reflective Fiber Surface Plasmon Resonance Sensor for High-Sensitive Mercury Ion Detection. Appl. Sci. 2019, 9, 1480. [Google Scholar] [CrossRef] [Green Version]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef]
- Kong, M.-R.; Zhang, B.-X.; Zhang, L.-L.; Jin, Y.-X.; Huan, Y.-S.; Shen, L.-G.; Yu, Q.-R. An ultrasensitive electrochemical “turn-on” label-free biosensor for Hg2+ with AuNP-functionalized reporter DNA as a signal amplifier. Chem. Commun. 2009, 37, 5633–5635. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Qian, S.; Guang, J.; Wang, J.; Han, X.; Masson, J.-F.; Peng, W. Mercaptopyridine-Functionalized Gold Nanoparticles for Fiber-Optic Surface Plasmon Resonance Hg2+ Sensing. ACS Sens. 2019, 4, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Lin, M.; Ji, W.; Yuan, H.; Zhang, Y.; Jing, Z.; Zhang, X.; Masson, J.-F.; Peng, W. Boronic Acid Functionalized Au Nanoparticles for Selective MicroRNA Signal Amplification in Fiber-Optic Surface Plasmon Resonance Sensing System. ACS Sens. 2018, 3, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Guang, J.; Sun, G.; Zhang, Y.; Han, X.; Masson, J.-F.; Peng, W. Au nanoparticles as label-free competitive reporters for sensitivity enhanced fiber-optic SPR heparin sensor. Biosens. Bioelectron. 2020, 154, 112039. [Google Scholar] [CrossRef]
- Chen, J.R.; Miao, Y.Q.; He, N.Y.; Wu, X.H.; Li, S.J. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar]
- Sagadevan, S.; Periasamy, M. Recent Trends in Nanobiosensors and Their Applications—A Review. Rev. Adv. Mater. Sci. 2014, 36, 62–69. [Google Scholar]
- Souza, L.R.R.; Zanatta, M.B.T.; da Silva, I.A.; da Veiga, M.A.M.S. Mercury determination in soil and sludge samples by HR CS GFAAS: Comparison of sample preparation procedures and chemical modifiers. J. Anal. Atom. Spectrom. 2018, 33, 1477–1485. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.J.; Luo, Z.W.; Liu, K.P.; Yang, X.J.; Zou, H.M.; Li, Y.X.; Duan, Y.X. Fiber-optic biosensor for detection of genetically modified food based on catalytic hairpin assembly reaction and nanocomposites assisted signal amplification. Sens. Actuators B Chem. 2018, 254, 956–965. [Google Scholar]
- Zeng, S.; Yu, X.; Law, W.-C.; Zhang, Y.; Hu, R.; Dinh, X.-Q.; Ho, H.-P.; Yong, K.-T. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sens. Actuators B Chem. 2013, 176, 1128–1133. [Google Scholar] [CrossRef]
- Lu, J.; Van Stappen, T.; Spasic, D.; Delport, F.; Vermeire, S.; Gils, A.; Lammertyn, J. Fiber optic-SPR platform for fast and sensitive infliximab detection in serum of inflammatory bowel disease patients. Biosens. Bioelectron. 2016, 79, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daems, D.; Lu, J.; Delport, F.; Mariën, N.; Orbie, L.; Aernouts, B.; Adriaens, I.; Huybrechts, T.; Saeys, W.; Spasic, D.; et al. Competitive inhibition assay for the detection of progesterone in dairy milk using a fiber-optic SPR biosensor. Anal. Chim. Acta 2017, 950, 1–6. [Google Scholar] [CrossRef]
- Ma, N.; Ren, X.; Wang, H.; Kuang, X.; Fan, D.; Wu, D.; Wei, Q. Ultrasensitive Controlled Release Aptasensor Using Thymine–Hg2+–Thymine Mismatch as a Molecular Switch for Hg2+ Detection. Anal. Chem. 2020, 92, 14069–14075. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, F.; Zhang, X.; Liu, Q.; Lu, M.; Ji, W.; Zhang, Y.; Jing, Z.; Peng, W. TFBG-SPR DNA-biosensor for Renewable Ultra-trace Detection of Mercury Ions. J. Light. Technol. 2020, 1. [Google Scholar] [CrossRef]
- Wang, N.; Lin, M.; Dai, H.; Ma, H. Functionalized gold nanoparticles/reduced graphene oxide nanocomposites for ultrasensitive electrochemical sensing of mercury ions based on thymine–mercury–thymine structure. Biosens. Bioelectron. 2016, 79, 320–326. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.-Y.; Yang, H.-F.; Shao, G.; Gan, F. Highly selective fluorescent carbon dots probe for mercury(ii) based on thymine–mercury(ii)–thymine structure. RSC Adv. 2018, 8, 3982–3988. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Dong, T.; Wang, Z.; Bao, J.; Tu, W.; Dai, Z. Aggregation of Individual Sensing Units for Signal Accumulation: Conversion of Liquid-Phase Colorimetric Assay into Enhanced Surface-Tethered Electrochemical Analysis. J. Am. Chem. Soc. 2015, 137, 8880–8883. [Google Scholar] [CrossRef]
- Xia, F.; Song, H.; Zhao, Y.; Zhao, W.-M.; Wang, Q.; Wang, X.-Z.; Wang, B.-T.; Dai, Z.-X. Ultra-high sensitivity SPR fiber sensor based on multilayer nanoparticle and Au film coupling enhancement. Measurement 2020, 164, 108083. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Boruah, B.S.; Ojah, N.; Biswas, R. Bio-Inspired Localized Surface Plasmon Resonance Enhanced Sensing of Mercury Through Green Synthesized Silver Nanoparticle. J. Light. Technol. 2020, 38, 2086–2091. [Google Scholar] [CrossRef]
- Prakashan, V.; Georgeab, G.; Sanu, M.S.; Sajna, M.S.; Saritha, A.; Sudarsanakumar, C.; Biju, P.; Josepha, C.; Unnikrishnan, N. Investigations on SPR induced Cu@Ag core shell doped SiO2-TiO2-ZrO2 fiber optic sensor for mercury detection. Appl. Surf. Sci. 2020, 507, 144957. [Google Scholar] [CrossRef]
- Taniguchi, M.; Siddiki, M.S.R.; Ueda, S.; Maeda, I. Mercury (II) sensor based on monitoring dissociation rate of the trans-acting factor MerR from cis-element by surface plasmon resonance. Biosens. Bioelectron. 2015, 67, 309–314. [Google Scholar] [CrossRef]
- Huang, D.; Hu, T.; Chen, N.; Zhang, W.; Di, J. Development of silver/gold nanocages onto indium tin oxide glass as a reagentless plasmonic mercury sensor. Anal. Chim. Acta 2014, 825, 51–56. [Google Scholar] [CrossRef]
- Shukla, G.M.; Punjabi, N.; Kundu, T.; Mukherji, S. Optimization of Plasmonic U-Shaped Optical Fiber Sensor for Mercury Ions Detection Using Glucose Capped Silver Nanoparticles. IEEE Sens. J. 2019, 19, 3224–3231. [Google Scholar] [CrossRef]










| Detection Method | Linear Range | LOD | Reference |
|---|---|---|---|
| SPR sensors | 0.01–0.5 ppm | 49.9 nM | [44] |
| Fiber-optic LSPR sensor | 30 to 200 μM | 30 μM | [25] |
| Fiber-optic LSPR sensor | 1 ppb to 15 ppb | 1.5 ppb | [45] |
| Fiber-optic SPR sensor | 0.01 to 1000 µM | 10 nM | [46] |
| SPR sensors | 1–25 µM | 1 µM | [24] |
| SPR sensors | 101–104 µg/L | 5 µg/L | [47] |
| SPR sensors | 0.01–5 ppm | 5 ppb | [48] |
| SPR sensors | 0–100 ppb | 2 ppb | [49] |
| Fiber-optic SPR sensor | 0–20 μM | 9.98 nM | This work |
| Sample | Hg2+ Added /nM | Hg2+ Found /nM | Recovery (%) Mean ± RSD, n = 3 |
|---|---|---|---|
| 1 | 80 | 86.1 ± 7.8 | 108 ± 10 |
| 2 | 100 | 97.4 ± 12.6 | 97 ± 12.6 |
| 3 | 200 | 211.7 ± 21.3 | 106 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Sun, G.; Peng, W.; Ji, W.; Chu, S.; Liu, Q.; Liang, Y. Thymine-Functionalized Gold Nanoparticles (Au NPs) for a Highly Sensitive Fiber-Optic Surface Plasmon Resonance Mercury Ion Nanosensor. Nanomaterials 2021, 11, 397. https://doi.org/10.3390/nano11020397
Yuan H, Sun G, Peng W, Ji W, Chu S, Liu Q, Liang Y. Thymine-Functionalized Gold Nanoparticles (Au NPs) for a Highly Sensitive Fiber-Optic Surface Plasmon Resonance Mercury Ion Nanosensor. Nanomaterials. 2021; 11(2):397. https://doi.org/10.3390/nano11020397
Chicago/Turabian StyleYuan, Huizhen, Guangyi Sun, Wei Peng, Wei Ji, Shuwen Chu, Qiang Liu, and Yuzhang Liang. 2021. "Thymine-Functionalized Gold Nanoparticles (Au NPs) for a Highly Sensitive Fiber-Optic Surface Plasmon Resonance Mercury Ion Nanosensor" Nanomaterials 11, no. 2: 397. https://doi.org/10.3390/nano11020397