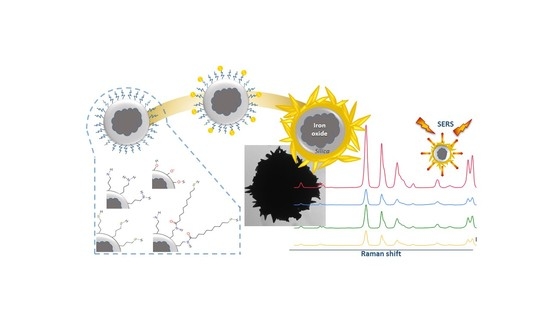
Nanostructured and Spiky Gold Shell Growth on Magnetic Particles for SERS Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Particle Synthesis
2.2.2. Surface Functionalization of Silica-coated Particles
2.2.3. Gold Growth on Functionalized Silica Surfaces
2.2.4. Techniques
3. Results and Discussion
3.1. Silica-Iron Oxide Core Functionalization
3.2. Gold Seeds Attachment
3.3. Spike Growth on Seeded Particles
3.4. Magnetic and SERS Properties of the Spiky Nanoparticles
3.5. Impact of the Growth Parameters on Nanoparticles’ Shape: Spiky vs Bumpy Nanoparticles
3.5.1. Elemental Composition of Spiky and Bumpy Nanoparticles
3.5.2. SERS Enhancement of Spiky and Bumpy Nanoparticles
3.5.3. Stability Overtime of Spiky and Bumpy Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelley, S.O.; Mirkin, C.A.; Walt, D.R.; Ismagilov, R.F.; Toner, M.; Sargent, E.H. Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat. Nanotechnol. 2014, 9, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walt, D.R. Miniature analytical methods for medical diagnostics. Science 2005, 308, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.Y.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.D.; Marquez, M.; Xia, Y.N. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Stewart, M.E.; Anderton, C.R.; Thompson, L.B.; Maria, J.; Gray, S.K.; Rogers, J.A.; Nuzzo, R.G. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef] [PubMed]
- Couture, M.; Zhao, S.S.; Masson, J.F. Modern surface plasmon resonance for bioanalytics and biophysics. Phys. Chem. Chem. Phys. 2013, 15, 11190–11216. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Roh, S.; Chung, T.; Lee, B. Overview of the Characteristics of Micro- and Nano-Structured Surface Plasmon Resonance Sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Salmain, M.; Liedberg, B.; Boujday, S. Naked Eye Immunosensing of Food Biotoxins Using Gold Nanoparticle-Antibody Bioconjugates. ACS Appl. Nano Mater. 2019, 2, 4150–4158. [Google Scholar] [CrossRef]
- Loiseau, A.; Zhang, L.; Hu, D.; Salmain, M.; Mazouzi, Y.; Flack, R.; Liedberg, B.; Boujday, S. Core-Shell Gold/Silver Nanoparticles for Localized Surface Plasmon Resonance-Based Naked-Eye Toxin Biosensing. ACS Appl. Mater. Interfaces 2019, 11, 46462–46471. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Salmain, M.; Boujday, S. Gold colloid-nanostructured surfaces for enhanced piezoelectric immunosensing of staphylococcal enterotoxin A. Sens. Actuators B 2018, 255, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Ben Haddada, M.; Hu, D.; Salmain, M.; Zhang, L.; Peng, C.; Wang, Y.; Liedberg, B.; Boujday, S. Gold nanoparticle-based localized surface plasmon immunosensor for staphylococcal enterotoxin A (SEA) detection. Anal. Bioanal. Chem. 2017, 409, 6227–6234. [Google Scholar] [CrossRef] [Green Version]
- Boujday, S.; de la Chapelle, M.L.; Srajer, J.; Knoll, W. Enhanced Vibrational Spectroscopies as Tools for Small Molecule Biosensing. Sensors 2015, 15, 21239–21264. [Google Scholar] [CrossRef]
- Pradier, C.M.; Salmain, M.; Boujday, S. Biointerface Characterization by Advanced IR Spectroscopy, 1st ed.; IR Spectroscopy for Biorecognition and Molecular Sensing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 167–216. [Google Scholar]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef]
- Darvill, D.; Centeno, A.; Xie, F. Plasmonic fluorescence enhancement by metal nanostructures: Shaping the future of bionanotechnology. Phys. Chem. Chem. Phys. 2013, 15, 15709–15726. [Google Scholar] [CrossRef]
- Dalstein, L.; Ben Haddada, M.; Barbillon, G.; Humbert, C.; Tadjeddine, A.; Boujday, S.; Busson, B. Revealing the Interplay between Adsorbed Molecular Layers and Gold Nanoparticles by Linear and Nonlinear Optical Properties. J. Phys. Chem. C 2015, 119, 17146–17155. [Google Scholar] [CrossRef] [Green Version]
- Dalstein, L.; Humbert, C.; Ben Haddada, M.; Boujday, S.; Barbillon, G.; Busson, B. The Prevailing Role of Hotspots in Plasmon-Enhanced Sum-Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2019, 10, 7706–7711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camden, J.P.; Dieringer, J.A.; Zhao, J.; Van Duyne, R.P. Controlled Plasmonic Nanostructures for Surface-Enhanced Spectroscopy and Sensing. Acc. Chem. Res. 2008, 41, 1653–1661. [Google Scholar] [CrossRef] [Green Version]
- Cialla, D.; Marz, A.; Bohme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Saada, T.N.; Meziane, D.; Boukherroub, R. Magneto-Optical Nanostructures for Viral Sensing. Nanomaterials 2020, 10, 1271. [Google Scholar] [CrossRef]
- Olsvik, O.; Popovic, T.; Skjerve, E.; Cudjoe, K.S.; Hornes, E.; Ugelstad, J.; Uhlen, M. Magnetic Separation Techniques in Diagnostic Microbiology. Clin. Microbiol. Rev. 1994, 7, 43–54. [Google Scholar] [CrossRef] [PubMed]
- De Las Cuevas, G.; Faraudo, J.; Camacho, J. Low-gradient magnetophoresis through field-induced reversible aggregation. J. Phys. Chem. C 2008, 112, 945–950. [Google Scholar] [CrossRef]
- Lim, J.; Majetich, S.A. Composite magnetic-plasmonic nanoparticles for biomedicine: Manipulation and imaging. Nano Today 2013, 8, 98–113. [Google Scholar] [CrossRef]
- Tartaj, P. Superparamagnetic Composites: Magnetism with No Memory. Eur. J. Inorg. Chem. 2009, 2009, 333–343. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Benelmekki, M.; Caparros, C.; Montras, A.; Goncalves, R.; Lanceros-Mendez, S.; Martinez, L.M. Horizontal low gradient magnetophoresis behaviour of iron oxide nanoclusters at the different steps of the synthesis route. J. Nanopart. Res. 2011, 13, 3199–3206. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Leshuk, T.; Everett, P.; Krishnakumar, H.; Wong, K.; Linley, S.; Gu, F. Mesoporous Magnetically Recyclable Photocatalysts for Water Treatment. J. Nanosci. Nanotechnol. 2013, 13, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Truby, R.L.; Emelianov, S.Y.; Homan, K.A. Ligand-Mediated Self-Assembly of Hybrid Plasmonic and Superparamagnetic Nanostructures. Langmuir 2013, 29, 2465–2470. [Google Scholar] [CrossRef]
- Hassan, N.; Cabuil, V.; Abou-Hassan, A. Assembling magneto-plasmonic microcapsules using a microfluidic device. Chem. Commun. 2013, 49, 412–414. [Google Scholar] [CrossRef]
- Wang, L.Y.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.H.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed core-shell Fe3O4@Au nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Xu, Z.C.; Hou, Y.L.; Sun, S.H. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Billen, A.; de Cattelle, A.; Jochum, J.K.; Van Bael, M.J.; Billen, J.; Seo, J.W.; Brullot, W.; Koeckelberghs, G.; Verbiest, T. Novel synthesis of superparamagnetic plasmonic core-shell iron oxide-gold nanoparticles. Phys. B Condens. Matter 2019, 560, 85–90. [Google Scholar] [CrossRef]
- Espinosa, A.; Bugnet, M.; Radtke, G.; Neveu, S.; Botton, G.A.; Wilhelm, C.; Abou-Hassan, A. Can magneto-plasmonic nanohybrids efficiently combine photothermia with magnetic hyperthermia? Nanoscale 2015, 7, 18872–18877. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured Surfaces and Assemblies as SERS Media. Small 2008, 4, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Crozier, K.B.; Zhu, W.Q.; Wang, D.X.; Lin, S.Y.; Best, M.D.; Camden, J.P. Plasmonics for Surface enhanced Raman Scattering: Nanoantennas for Single Molecules. IEEE J Sel. Top. Quant. 2014, 20, 152–162. [Google Scholar] [CrossRef]
- Li, W.Y.; Camargo, P.H.C.; Lu, X.M.; Xia, Y.N. Dimers of Silver Nanospheres: Facile Synthesis and Their Use as Hot Spots for Surface-Enhanced Raman Scattering. Nano Lett. 2009, 9, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Lee, W.; Kim, Y.; Kim, D. Self-aligned colocalization of 3D plasmonic nanogap arrays for ultra-sensitive surface plasmon resonance detection. Biosens. Bioelectron. 2014, 51, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sauerbeck, C.; Haderlein, M.; Schurer, B.; Braunschweig, B.; Peukert, W.; Taylor, R.N.K. Shedding Light on the Growth of Gold Nanoshells. ACS Nano 2014, 8, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Aldeanueva-Potel, P.; Carbo-Argibay, E.; Pazos-Perez, N.; Barbosa, S.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Spiked Gold Beads as Substrates for Single-Particle SERS. ChemPhysChem 2012, 13, 2561–2565. [Google Scholar] [CrossRef]
- Esenturk, E.N.; Walker, A.R.H. Surface-enhanced Raman scattering spectroscopy via gold nanostars. J. Raman Spectrosc. 2009, 40, 86–91. [Google Scholar] [CrossRef]
- Hrelescu, C.; Sau, T.K.; Rogach, A.L.; Jackel, F.; Feldmann, J. Single gold nanostars enhance Raman scattering. Appl. Phys. Lett. 2009, 94, 153113. [Google Scholar] [CrossRef]
- Osinkina, L.; Lohmuller, T.; Jackel, F.; Feldmann, J. Synthesis of Gold Nanostar Arrays as Reliable, Large-Scale, Homogeneous Substrates for Surface-Enhanced Raman Scattering Imaging and Spectroscopy. J. Phys. Chem. C 2013, 117, 22198–22202. [Google Scholar] [CrossRef]
- Lal, S.; Grady, N.K.; Kundu, J.; Levin, C.S.; Lassiter, J.B.; Halas, N.J. Tailoring plasmonic substrates for surface enhanced spectroscopies. Chem. Soc. Rev. 2008, 37, 898–911. [Google Scholar] [CrossRef]
- Bedford, E.E.; Boujday, S.; Pradier, C.M.; Gu, F.X. Nanostructured and spiky gold in biomolecule detection: Improving binding efficiencies and enhancing optical signals. RSC Adv. 2015, 5, 16461–16475. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Zhang, X.; Liu, Y.; Zhou, D.; Sun, H.Z.; Zhang, H.; Yang, B. Controllable Synthesis of Stable Urchin-like Gold Nanoparticles Using Hydroquinone to Tune the Reactivity of Gold Chloride. J. Phys. Chem. C 2011, 115, 3630–3637. [Google Scholar] [CrossRef]
- Miao, X.M.; Wang, T.T.; Chai, F.; Zhang, X.L.; Wang, C.G.; Sun, W.D. A facile synthetic route for the preparation of gold nanostars with magnetic cores and their reusable nanohybrid catalytic properties. Nanoscale 2011, 3, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, P.; Osorio, I.; Doria, G.; Carvalho, P.A.; Pereira, A.; Langer, J.; Araujo, J.P.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Franco, R.; et al. Star-shaped magnetite@gold nanoparticles for protein magnetic separation and SERS detection. RSC Adv. 2014, 4, 3659–3667. [Google Scholar] [CrossRef]
- Song, H.M.; Wei, Q.S.; Ong, Q.K.; Wei, A. Plasmon-Resonant Nanoparticles and Nanostars with Magnetic Cores: Synthesis and Magnetomotive Imaging. ACS Nano 2010, 4, 5163–5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.S.; Song, H.M.; Leonov, A.P.; Hale, J.A.; Oh, D.M.; Ong, Q.K.; Ritchie, K.; Wei, A. Gyromagnetic Imaging: Dynamic Optical Contrast Using Gold Nanostars with Magnetic Cores. J. Am. Chem. Soc. 2009, 131, 9728–9734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedford, E.E.; Boujday, S.; Pradier, C.M.; Gu, F.X. Spiky gold shells on magnetic particles for DNA biosensors. Talanta 2018, 182, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Ben Haddada, M.; Blanchard, J.; Casale, S.; Krafft, J.M.; Vallee, A.; Methivier, C.; Boujday, S. Optimizing the immobilization of gold nanoparticles on functionalized silicon surfaces: Amine- vs thiol-terminated silane. Gold Bull. 2013, 46, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Boujday, S.; Lambert, J.F.; Che, M. Amorphous silica as a versatile supermolecular ligand for Ni-II amine complexes: Toward interfacial molecular recognition. ChemPhysChem 2004, 5, 1003–1013. [Google Scholar] [CrossRef]
- Hervier, A.; Blanchard, J.; Costentin, G.; Regalbuto, J.; Louis, C.; Boujday, S. The genesis of a heterogeneous catalyst: In situ observation of a transition metal complex adsorbing onto an oxide surface in solution. Chem. Commun. 2014, 50, 2409–2411. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Philipse, A.P.; Vanbruggen, M.P.B.; Pathmamanoharan, C. Magnetic silica dispersions - preparation and stability of surface-modified silica particles with a magnetic core. Langmuir 1994, 10, 92–99. [Google Scholar] [CrossRef]
- Dendramis, A.L.; Schwinn, E.W.; Sperline, R.P. A surface-enhanced Raman scattering study of CTAB adsorption on copper. Surf. Sci. 1983, 134, 675–688. [Google Scholar] [CrossRef]
- Lee, S.; Anderson, L.J.; Payne, C.M.; Hafner, J.H. Structural transition in the surfactant layer that surrounds gold nanorods as observed by analytical surface-enhanced Raman spectroscopy. Langmuir 2011, 27, 14748–14756. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Mao, B.W.; Tian, Z.Q. Surface-Enhanced Raman-Spectroscopic Studies on Silver Electrodes in the Presence of 2-Mercaptopyrimidine. J. Raman Spectrosc. 1995, 26, 233–237. [Google Scholar] [CrossRef]
- Murphy, C.J.; San, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.X.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Romo-Herrera, J.M.; Perez-Juste, J.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Reshaping and LSPR tuning of Au nanostars in the presence of CTAB. J. Mater. Chem. 2011, 21, 11544–11549. [Google Scholar] [CrossRef]
- Liu, M.Z.; Guyot-Sionnest, P. Mechanism of silver(I)-assisted growth of gold nanorods and bipyramids. J. Phys. Chem. B 2005, 109, 22192–22200. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Qian, Z.X.; Hastings, S.P.; Reca, M.L.; Fakhraai, Z.; Park, S.J. Controlling the Topography and Surface Plasmon Resonance of Gold Nanoshells by a Templated Surfactant-Assisted Seed Growth Method. J. Phys. Chem. C 2013, 117, 8916–8923. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Chen, C.H.; Huang, M.H. Seed-Mediated Synthesis of Branched Gold Nanocrystals Derived from the Side Growth of Pentagonal Bipyramids and the Formation of Gold Nanostars. Chem. Mater. 2009, 21, 110–114. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, T.; Hussain, J.I.; Hashmi, A.A. Au(III)-CTAB reduction by ascorbic acid: Preparation and characterization of gold nanoparticles. Colloids Surf., B 2013, 104, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Trigari, S.; Rindi, A.; Margheri, G.; Sottini, S.; Dellepiane, G.; Giorgetti, E. Synthesis and modelling of gold nanostars with tunable morphology and extinction spectrum. J. Mater. Chem. 2011, 21, 6531–6540. [Google Scholar] [CrossRef] [Green Version]
- Vega, M.M.; Bonifacio, A.; Lughi, V.; Marsi, S.; Carrato, S.; Sergo, V. Long-term stability of surfactant-free gold nanostars. J. Nanopart. Res. 2014, 16, 2729. [Google Scholar] [CrossRef]
- Bassi, B.; Albini, B.; D’Agostino, A.; Dacarro, G.; Pallavicini, P.; Galinetto, P.; Taglietti, A. Robust, reproducible, recyclable SERS substrates: Monolayers of gold nanostars grafted on glass and coated with a thin silica layer. Nanotechnology 2019, 30, 025302. [Google Scholar] [CrossRef]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Silica-Coated Gold Nanostars for Combined Surface-Enhanced Raman Scattering (SERS) Detection and Singlet-Oxygen Generation: A Potential Nanoplatform for Theranostics. Langmuir 2011, 27, 12186–12190. [Google Scholar] [CrossRef] [Green Version]
- Montoto, A.H.; Montes, R.; Samadi, A.; Gorbe, M.; Terres, J.M.; Cao-Milan, R.; Aznar, E.; Ibanez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser Induced Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef]








| Surface | Si2p | O1s | C1s | N1s | S2s | C/Si | O/Si | N/Si × 100 | S/Si × 100 |
|---|---|---|---|---|---|---|---|---|---|
| Bare | 33.8 | 56.6 | 9.0 | 0.59 | - | 0.27 | 1.62 | 1.7 | - |
| SH | 33.6 | 55.4 | 10.7 | - | 0.28 | 0.32 | 1.65 | - | 0.83 |
| NH2 | 30.1 | 46.0 | 21.3 | 2.59 | - | 0.71 | 1.53 | 8.6 | - |
| SH-NH2 | 27.5 | 44.4 | 25.1 | 2.63 | 0.34 | 0.91 | 1.62 | 9.6 | 1.24 |
| Shape | Si2p | O1s | Au4f | Ag3d | Br3d | Au/Si | Ag/Si | Br/Si | Br/Au |
|---|---|---|---|---|---|---|---|---|---|
| Spiky | 15.9 | 22.2 | 7.7 | 1.7 | 1.0 | 0.48 | 0.11 | 0.06 | 0.13 |
| Bumpy | 7.6 | 14.1 | 10.8 | 2.4 | 1.3 | 1.42 | 0.31 | 0.18 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedford, E.E.; Méthivier, C.; Pradier, C.-M.; Gu, F.; Boujday, S. Nanostructured and Spiky Gold Shell Growth on Magnetic Particles for SERS Applications. Nanomaterials 2020, 10, 2136. https://doi.org/10.3390/nano10112136
Bedford EE, Méthivier C, Pradier C-M, Gu F, Boujday S. Nanostructured and Spiky Gold Shell Growth on Magnetic Particles for SERS Applications. Nanomaterials. 2020; 10(11):2136. https://doi.org/10.3390/nano10112136
Chicago/Turabian StyleBedford, Erin E., Christophe Méthivier, Claire-Marie Pradier, Frank Gu, and Souhir Boujday. 2020. "Nanostructured and Spiky Gold Shell Growth on Magnetic Particles for SERS Applications" Nanomaterials 10, no. 11: 2136. https://doi.org/10.3390/nano10112136