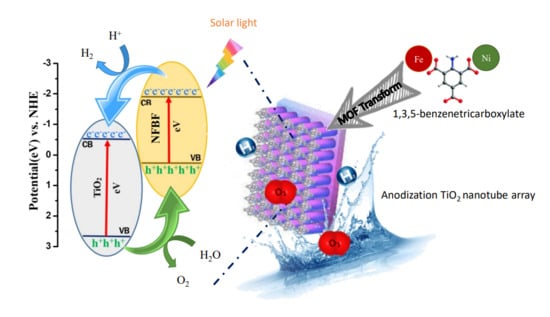
Fe/Ni Bimetallic Organic Framework Deposited on TiO2 Nanotube Array for Enhancing Higher and Stable Photoelectrochemical Activity of Oxygen Evaluation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production of TiO2 Nanotube Array Electrodes on Ti Foils
2.3. Electrodeposition of Ni on TNTA and Bimetallic Fe/Ni on TNTA
2.4. Transformation of Bimetallic FeNi-MOF/TNTA from FeNi/TNTA
2.5. Characterization
2.6. Photoelectrochemical Measurements
3. Results and Discussion
3.1. Structural and Morphological Characterization of FeNi-MOF/TNTA
3.2. Photoelectrochemical Analysis of FeNi-MOF/TNTA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef] [PubMed]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Ledezma-Yanez, I.; Koper, M.T.M.; Calle-Vallejo, F. Guidelines for the rational design of Ni-based double hydroxide electrocatalysts for the oxygen evolution reaction. ACS Catal. 2015, 5, 5380–5387. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.-J.; Sokaras, D.; Weng, T.-C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni, Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef]
- Matsuda, S.; Kato, A. Titanium oxide based catalysts—A review. Appl. Catal. 1983, 8, 149–165. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (anatase and rutile): Surface chemistry, liquid-solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Yang, Y.; Dai, X.; Zhao, H.; Yong, J.; Yu, L.; Luan, X.; Cui, M.; Zhang, X.; Huang, X. Amorphous (Fe)Ni-MOF-derived hollow (bi)metal/oxide@N-graphene polyhedron as effectively bifunctional catalysts in overall alkaline water splitting. Electrochim. Acta 2019, 318, 430–439. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, W.; Jiao, L.; Yu, S.-H.; Jiang, H.-L. Template-Directed Growth of Well-Aligned MOF Arrays and Derived Self-Supporting Electrodes for Water Splitting. Chemistry 2017, 2, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef] [PubMed]
- Skorupskii, G.; Trump, B.A.; Kasel, T.W.; Brown, C.M.; Hendon, C.H.; Dincă, M. Efficient and tunable one-dimensional charge transport in layered lanthanide metal–organic frameworks. Nat. Chem. 2020, 12, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kwak, J.H.; Choe, W. Evolution of form in metal-organic frameworks. Nat. Commun. 2017, 8, 14070. [Google Scholar] [CrossRef] [Green Version]
- Doonan, C.J.; Sumby, C.J. Metal-organic framework catalysis. CrystEngComm 2017, 19, 4044–4048. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Vlasova, E.A.; Yakimov, S.A.; Naidenko, E.V.; Kudrik, E.V.; Makarov, S.V. Application of metal-organic frameworks for purification of vegetable oils. Food. Chem. 2016, 190, 103–109. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- El Rouby, W.M.A.; Antuch, M.; You, S.M.; Beaunier, P.; Millet, P. Novel nano-architectured water splitting photoanodes based on TiO2-nanorod mats surface sensitized by ZIF-67 coatings. Int. J. Hydrogen Energy 2019, 44, 30949–30964. [Google Scholar] [CrossRef]
- Xing, J.; Guo, K.; Zou, Z.; Cai, M.; Du, J.; Xu, C. In situ growth of well-ordered NiFe-MOF-74 on Ni foam by Fe2+ induction as an efficient and stable electrocatalyst for water oxidation. Chem. Commun. 2018, 54, 7046–7049. [Google Scholar] [CrossRef]
- Fan, C.; Dong, H.; Liang, Y.; Yang, J.; Tang, G.; Zhang, W.; Cao, Y. Sustainable synthesis of HKUST-1 and its composite by biocompatible ionic liquid for enhancing visible-light photocatalytic performance. J. Cleaner Product. 2019, 208, 353–362. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chen, C.; Ahn, W.-S. Chromium terephthalate metal–organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef] [Green Version]
- Cardenas-Morcoso, D.; Ifraemov, R.; García-Tecedor, M.; Liberman, I.; Gimenez, S.; Hod, I. A metal–organic framework converted catalyst that boosts photo-electrochemical water splitting. J. Mater. Chem. A 2019, 7, 11143–11149. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The recent development of efficient Earth-abundant transition-metal nanocatalysts. Chem. Soc. Rev. 2017, 46, 816–854. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization Fabrication of Highly Ordered TiO2 Nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Doong, R.A.; Liao, C.Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method. J. Hazard. Mater. 2017, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Dong, W.; Li, H.; Xi, J.; Mu, J.; Huang, Y.; Ji, Z.; Wu, X. Reduced TiO2 nanoflower structured photoanodes for superior photoelectrochemical water splitting. J. Alloys Compd. 2017, 724, 280–286. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.A. Photocatalytic degradation of bisphenol A over a p-n heterojunction of ZnFe2O4/TiO2 under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef]
- Chiang, L.F.; Doong, R.A. Cu-TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol a degradation. J. Hazard. Mater. 2014, 277, 84–92. [Google Scholar] [CrossRef]
- Yang, Q.; Li, T.; Lu, Z.; Sun, X.; Liu, J. Hierarchical construction of an ultrathin layered double hydroxide nanoarray for highly-efficient oxygen evolution reaction. Nanoscale 2014, 6, 11789–11794. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Wang, M.; Sheng, X.; Chen, X.; Feng, X.; Mao, S.S. Titanium dioxide nanostructures for photoelectrochemical applications. Prog. Mater. Sci. 2018, 98, 299–385. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Mohapatra, S.K.; Misra, M. Stability of TiO2 nanotube arrays in photoelectrochemical studies. Int. J. Hydrogen Energy 2008, 33, 5369–5374. [Google Scholar] [CrossRef]
- Su, F.; Lu, J.; Tian, Y.; Ma, X.; Gong, J. Branched TiO2 nanoarrays sensitized with CdS quantum dots for highly efficient photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2013, 15, 12026–12032. [Google Scholar] [CrossRef]
- Choi, I.; Jung, Y.E.; Yoo, S.J.; Kim, J.Y.; Kim, H.-J.; Lee, C.Y.; Jang, J.H. Facile synthesis of M-MOF-74 (M = Co, Ni, Zn) and its application as an electrocatalyst for electrochemical CO2 sonversion and H2 production. J. Electrochem. Sci. Technol. 2017, 8, 61–68. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Jimoh, A.A.; Shaikh, M.N.; Ali, B.E. Mixed-metalmetal–organic frameworks as catalysts for liquid-phase oxidation of toluene and cycloalkanes. Arab. J. Sci. Eng. 2017, 42, 4383–4390. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. Metal–organic-framework-derived Co–Fe bimetallic oxygen reduction electrocatalysts for alkaline fuel cells. J. Am. Chem. Soc. 2019, 141, 10744–10750. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Song, K.S.; Buyukcakir, O.; Yildirim, T.; Coskun, A. Bimetallic metal organic frameworks with precisely positioned metal centers for efficient H2 storage. Chem. Commun. 2018, 54, 12218–12221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Lee, S.; Oh, S.; Park, H.; Choi, S.; Oh, M. Synthesis of bimetallic conductive 2D metal–organic framework (CoxNiy-CAT) and its mass production: Enhanced electrochemical oxygen reduction activity. Small 2019, 15, 1805232. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, D.-R.; Wang, W.-N. Bimetallic metal-organic frameworks (MOFs) synthesized using the spray method for tunable CO2 adsorption. Chem. Eng. J. 2020, 382, 122825. [Google Scholar] [CrossRef]
- Cui, W.; Bai, H.; Shang, J.; Wang, F.; Xu, D.; Ding, J.; Fan, W.; Shi, W. Organic-inorganic hybrid-photoanode built from NiFe-MOF and TiO2 for efficient PEC water splitting. Electrochim. Acta 2020, 349, 136383. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Xu, S.; Fu, Y.; Zhang, R.; Wei, M.; Evans, D.G.; Duan, X. TiO2/graphene/NiFe-layered double hydroxide nanorod array photoanodes for efficient photoelectrochemical water splitting. Energy Environ. Sci. 2016, 9, 2633–2643. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Jagadale, A.D.; Hao, X.; Abudula, A.; Guan, G. Fabrication of Cu(OH)2@NiFe-layered double hydroxide catalyst array for electrochemical water splitting. Int. J. Hydrogen Energy 2016, 41, 14553–14561. [Google Scholar] [CrossRef]
- Li, A.L.; Wang, Z.L.; Yin, H.; Wang, S.Y.; Yan, P.L.; Huang, B.K.; Wang, X.L.; Li, R.G.; Zong, X.; Han, H.X.; et al. Understanding the anatase-rutile phase junction in charge separation and transfer in a TiO2 electrode for photoelectrochemical water splitting. Chem. Sci. 2016, 7, 6076–6082. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Wu, Z.; Tong, Y.; Wu, Z.; Cravotto, G. Microwave-assisted synthesis of carbon-based (N, Fe)-codoped TiO2 for the photocatalytic degradation of formaldehyde. Nanoscale Res. Lett. 2015, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Kottegoda, I.R.M.; Idris, N.H.; Lu, L.; Wang, J.-Z.; Liu, H.-K. Synthesis and characterization of graphene–nickel oxide nanostructures for fast charge–discharge application. Electrochim. Acta 2011, 56, 5815–5822. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Liu, B.; Zhao, Q.; Chen, G. Hexagonal microspindle of NH2-MIL-101(Fe) metal–organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene. RSC Adv. 2016, 6, 4289–4295. [Google Scholar] [CrossRef]
- El Rouby, W.M.A.; Antuch, M.; You, S.M.; Beaunier, P.; Millet, P. Surface sensitization of TiO2 nanorod mats by electrodeposition of ZIF67 for water photo-oxidation. Electrochim. Acta 2020, 339, 135882. [Google Scholar]
- Yoshio, S.; Maki, K. Computational modeling of the effect of varying aqueous solutions on Ni(OH)2 precipitates. AIP Adv. 2018, 8, 025217. [Google Scholar] [CrossRef] [Green Version]
- González-Flores, D.; Klingan, K.; Chernev, P.; Loos, S.; Mohammadi, M.R.; Pasquini, C.; Kubella, P.; Zaharieva, I.; Smith, R.D.L.; Dau, H. Nickel-iron catalysts for electrochemical water oxidation–redox synergism investigated by in situ X-ray spectroscopy with millisecond time resolution. Sustain. Energy Fuels 2018, 2, 1986–1994. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.-M.; El Rouby, W.M.A.; Thamilselvan, A.; Tsai, C.-K.; Darmanto, W.; Doong, R.-A.; Millet, P. Fe/Ni Bimetallic Organic Framework Deposited on TiO2 Nanotube Array for Enhancing Higher and Stable Photoelectrochemical Activity of Oxygen Evaluation Reaction. Nanomaterials 2020, 10, 1688. https://doi.org/10.3390/nano10091688
You S-M, El Rouby WMA, Thamilselvan A, Tsai C-K, Darmanto W, Doong R-A, Millet P. Fe/Ni Bimetallic Organic Framework Deposited on TiO2 Nanotube Array for Enhancing Higher and Stable Photoelectrochemical Activity of Oxygen Evaluation Reaction. Nanomaterials. 2020; 10(9):1688. https://doi.org/10.3390/nano10091688
Chicago/Turabian StyleYou, Sheng-Mu, Waleed M. A. El Rouby, Annadurai Thamilselvan, Cheng-Kuo Tsai, Win Darmanto, Ruey-An Doong, and Pierre Millet. 2020. "Fe/Ni Bimetallic Organic Framework Deposited on TiO2 Nanotube Array for Enhancing Higher and Stable Photoelectrochemical Activity of Oxygen Evaluation Reaction" Nanomaterials 10, no. 9: 1688. https://doi.org/10.3390/nano10091688