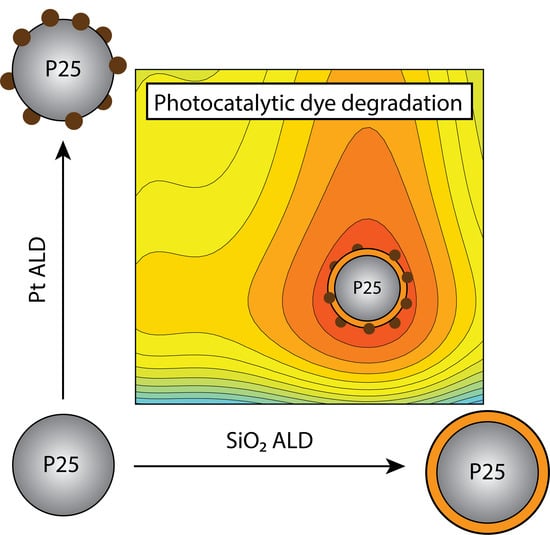
Synthesis of a Rationally Designed Multi-Component Photocatalyst Pt:SiO2:TiO2(P25) with Improved Activity for Dye Degradation by Atomic Layer Deposition
Abstract
:1. Introduction
- (1)
- Adsorption of reactants.
- (2)
- Creation of charge carriers by light absorption.
- (3)
- Charge carrier separation.
- (4)
- Degradation reactions, along:
- (a)
- Conduction band pathway via superoxide radicals (converting into hydroxyl radicals).
- (b)
- Valence band pathway via direct oxidation.
- (c)
- Valence band pathway via hydroxyl radicals.
2. Experimental Section
2.1. Synthesis
- (1)
- SiO2 was deposited on TiO2(P25) on a homebuilt ALD setup in a fluidized bed under atmospheric pressure, as described in detail elsewhere [36,37] In brief, 5 g of the TiO2(P25) powder was put in a quartz glass column (diameter 26 mm, height 500 mm), which was then placed on a vertical vibration table (Paja 40/40-24, Oosterhout, The Netherlands) to assist fluidization. The powder was sieved prior to the ALD experiments with a mesh size of 250 µm to break or exclude larger agglomerates. SiO2 layers were deposited using SiCl4 and H2O as precursors, which were both kept at room temperature in stainless steel bubblers. The reactor was heated to 100 °C throughout the deposition process using an IR lamp. For different SiO2 loadings, up to 40 cycles were applied using an exposure time of 30 s SiCl4 and a 3 min H2O pulse. Purging steps of nitrogen for 3 min and 8 min, respectively, separated the precursor pulses.
- (2)
- The SiO2:TiO2(P25)powder was split into 1.5 g batches, which were then used for the deposition of Pt clusters on the SiO2:TiO2(P25)surface using MeCpPtMe3and O2 as precursors. The Pt precursor was stored in stainless steel bubblers and held at 70 °C. For those experiments, one ALD cycle was performed using exposure times for the Pt precursor ranging from 20 sec to 5 min. The O2 exposure was set to 5 min, and both precursor exposures were separated using purge steps of 5 min with Nitrogen, respectively. Afterward the coated powders were treated under an atmosphere of 5% H2 in N2 (v/v) in a fixed bed reactor. The temperature was ramped up from room temperature to 200 °C with a rate of 2 °C/min and then was held constant for 5 min after which the powder was allowed to cool to room temperature.
2.2. Characterization
2.3. Photocatalytic Testing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.; Buijnsters, J.G.; Figueiredo, J.L.; Silva, A.M.; Faria, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- Driessen, M.D.; Grassian, V.H. Photooxidation of Trichloroethylene on Pt/TiO2. J. Phys. Chem. B 1998, 102, 1418–1423. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water Air Soil Pollut. 2010, 215, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.; Hamilton, J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Carneiro, J.T.; Savenije, T.J.; Moulijn, J.A.; Mul, G. The effect of Au on TiO2 catalyzed selective photocatalytic oxidation of cyclohexane. J. Photochem. Photobiol. A 2011, 217, 326–332. [Google Scholar] [CrossRef]
- Lim, T.H.; Jeong, S.M.; Kim, S.D.; Gyenis, J. Degradation characteristics of NO by photocatalysis with TiO2 and CuO/TiO2. React. Kinet. Catal. Lett. 2000, 71, 223–229. [Google Scholar] [CrossRef]
- Lei, M.; Wang, N.; Zhu, L.H.; Zhou, Q.L.; Nie, G.; Tang, H.Q. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: A mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper. Appl. Catal. B Environ. 2016, 182, 414–423. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, D.P.; Wu, R.; Gazi, S.; Soo, H.S.; Sritharan, T.; Chen, Z. New insights into the photocatalytic activity of 3-D core–shell P25@silica nanocomposites: Impact of mesoporous coating. Dalton Trans. 2017, 46, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; King, D.M.; Liang, X.; Li, J.; Weimer, A.W. Optimal preparation of Pt/TiO2 photocatalysts using atomic layer deposition. Appl. Catal. B 2010, 101, 54–60. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef] [Green Version]
- Muhich, C.L.; Zhou, Y.; Holder, A.M.; Weimer, A.W.; Musgrave, C.B. Effect of Surface Deposited Pt on the Photoactivity of TiO2. J. Phys. Chem. C 2012, 116, 10138–10149. [Google Scholar] [CrossRef]
- Benz, D.; Bui, H.V.; Hintzen, H.T.; Kreutzer, M.T.; Van Ommen, J.R. Mechanistic insight into the improved photocatalytic degradation of dyes using TiO2(P25) nanoparticles with an ultrathin SiO2 coating. In press.
- Guo, J.; Benz, D.; Nguyen, T.-T.D.; Nguyen, P.-H.; Le, T.-L.T.; Nguyen, H.-H.; La Zara, D.; Liang, B.; Hintzen, H.B.; Van Ommen, J.R.; et al. Tuning the Photocatalytic Activity of TiO2 Nanoparticles by Ultrathin SiO2 Films Grown by Low-Temperature Atmospheric Pressure Atomic Layer Deposition. Appl. Surf. Sci. 2020, 530, 147244. [Google Scholar] [CrossRef]
- Gaya, U.I. Mechanistic Principles of Photocatalytic Reaction. In Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Gaya, U.I., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 73–89. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Zhang, A.-Y.; Wang, W.-K.; Pei, D.-N.; Yu, H.-Q. Degradation of refractory pollutants under solar light irradiation by a robust and self-protected ZnO/CdS/TiO2 hybrid photocatalyst. Water Res. 2016, 92, 78–86. [Google Scholar]
- Yu, X.; Liu, S.; Yu, J. Superparamagnetic γ-Fe2O3@SiO2@TiO2 composite microspheres with superior photocatalytic properties. Appl. Catal. B 2011, 104, 12–20. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Liu, Y.; Wang, J.; Ji, G. Fabrication and enhanced photocatalytic properties of Pt@SiO2@TiO2 composites by surface plasma resonance from Pt nanoparticles. J. Nanopart. Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Li, S.-X.; Cai, J.; Wu, X.; Liu, B.; Chen, Q.; Li, Y.; Zheng, F. TiO2@Pt@CeO2 nanocomposite as a bifunctional catalyst for enhancing photo-reduction of Cr (VI) and photo-oxidation of benzyl alcohol. J. Hazard. Mater. 2018, 346, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, S. Vapor phase synthesis of 2,3,6-trimethylphenol from m-cresol and methanol with Fe2O3-SiO2-CuO catalyst. Catal. Commun. 2018, 111, 100–103. [Google Scholar] [CrossRef]
- Wang, J.; Kondrat, S.; Wang, Y.; Brett, G.L.; Giles, C.; Bartley, J.K.; Lu, L.; Liu, Q.; Kiely, C.J.; Hutchings, G.J. Au-Pd Nanoparticles Dispersed on Composite Titania/Graphene Oxide-Supports as a Highly Active Oxidation Catalyst. ACS Catal. 2015, 5, 3575–3587. [Google Scholar] [CrossRef]
- Liu, S.; Guo, M.-X.; Shao, F.; Peng, Y.-H.; Bian, S.-W. Water-dispersible and magnetically recoverable Fe3O4/Pd@nitrogen-doped carbon composite catalysts for the catalytic reduction of 4-nitrophenol. RSC Adv. 2016, 6, 76128–76131. [Google Scholar] [CrossRef]
- Cheng, N.; Banis, M.N.; Liu, J.; Riese, A.; Li, X.; Li, R.; Ye, S.; Knights, S.; Sun, X. Extremely Stable Platinum Nanoparticles Encapsulated in a Zirconia Nanocage by Area-Selective Atomic Layer Deposition for the Oxygen Reduction Reaction. Adv. Mater. 2015, 27, 277–281. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, M.; Wang, X.; Wu, Y.; Huang, C.; Fu, W.; Meng, X.; Sun, Y. Visible-light promoted catalytic activity of dumbbell-like Au nanorods supported on graphene/TiO2 sheets towards hydrogenation reaction. Nanotechnology 2018, 29, 245703. [Google Scholar] [CrossRef]
- Bo, Z.; Ahn, S.; Ardagh, M.A.; Schweitzer, N.M.; Canlas, C.P.; Farha, O.K.; Notestein, J.M.; Farh, O.K. Synthesis and stabilization of small Pt nanoparticles on TiO2 partially masked by SiO2. Appl. Catal. A 2018, 551, 122–128. [Google Scholar] [CrossRef]
- Song, Z.; Wang, B.; Cheng, N.; Yang, L.; Banham, D.; Li, R.; Ye, S.; Sun, X. Atomic layer deposited tantalum oxide to anchor Pt/C for a highly stable catalyst in PEMFCs. J. Mater. Chem. A 2017, 5, 9760–9767. [Google Scholar] [CrossRef]
- Wan, C.; Cheng, D.G.; Chen, F.; Zhan, X. Fabrication of CeO2 nanotube supported Pt catalyst encapsulated with silica for high and stable performance. Chem. Commun. 2015, 51, 9785–9788. [Google Scholar] [CrossRef]
- Zhao, E.W.; Maligal-Ganesh, R.V.; Mentink-Vigier, F.; Zhao, T.Y.; Du, Y.; Pei, Y.; Huang, W.; Bowers, C.R. Atomic-Scale Structure of Mesoporous Silica-Encapsulated Pt and PtSn Nanoparticles Revealed by Dynamic Nuclear Polarization-Enhanced 29Si MAS NMR Spectroscopy. J. Phys. Chem. C 2019, 123, 7299–7307. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Liu, W.; Zhang, T.G.; Stavropoulos, P.; Levy, B. Role of Photoinduced Charge Carrier Separation Distance in Heterogeneous Photocatalysis: Oxidative Degradation of CH3OH Vapor in Contact with Pt/TiO2 and Cofumed TiO2-Fe2O3. J. Phys. Chem. 1996, 100, 19466–19474. [Google Scholar] [CrossRef]
- Chen, J.-J.; Wang, W.-K.; Li, W.-W.; Pei, D.-N.; Yu, H.-Q. Roles of Crystal Surface in Pt-Loaded Titania for Photocatalytic Conversion of Organic Pollutants: A First-Principle Theoretical Calculation. ACS Appl. Mater. Interfaces 2015, 7, 12671–12678. [Google Scholar] [CrossRef] [PubMed]
- Benz, D.; Felter, K.M.; Köser, J.; Thöming, J.; Mul, G.; Grozema, F.C.; Hintzen, H.T.; Kreutzer, M.T.; van Ommen, J.R. Assessing the Role of Pt Clusters on TiO2(P25) on the Photocatalytic Degradation of Acid Blue 9 and Rhodamine B. J. Phys. Chem. C 2020, 124, 8269–8278. [Google Scholar] [CrossRef] [Green Version]
- Beetstra, R.; Lafont, U.; Nijenhuis, J.; Kelder, E.M.; van Ommen, J.R. Atmospheric Pressure Process for Coating Particles Using Atomic Layer Deposition. Chem. Vap. Depos. 2009, 15, 227–233. [Google Scholar] [CrossRef]
- van Ommen, J.R.; Goulas, A. Atomic layer deposition on particulate materials. Mater. Today Chem. 2019, 14, 100183. [Google Scholar] [CrossRef]
- van Driel, B.A.; Kooyman, P.J.; van den Berg, K.J.; Schmidt-Ott, A.; Dik, J. A quick assessment of the photocatalytic activity of TiO2 pigments—From lab to conservation studio! Microchem. J. 2016, 126, 162–171. [Google Scholar] [CrossRef]
- Shan, J.; Lei, Z.; Wu, W.; Tan, Y.; Cheng, N.; Sun, X. Highly Active and Durable Ultrasmall Pd Nanocatalyst Encapsulated in Ultrathin Silica Layers by Selective Deposition for Formic Acid Oxidation. ACS Appl. Mater. Interfaces 2019, 11, 43130–43137. [Google Scholar] [CrossRef]
- Faust, M.; Enders, M.; Gao, K.; Reichenbach, L.; Muller, T.; Gerlinger, W.; Sachweh, B.; Kasper, G.; Bruns, M.; Bräse, S.; et al. Synthesis of Pt/SiO2 Catalyst Nanoparticles from a Continuous Aerosol Process using Novel Cyclo-octadienylplatinum Precursors. Chem. Vap. Depos. 2013, 19, 274–283. [Google Scholar] [CrossRef]
- Gao, B.; Ma, Y.; Cao, Y.; Yang, W.; Yao, J. Great Enhancement of Photocatalytic Activity of Nitrogen-Doped Titania by Coupling with Tungsten Oxide. J. Phys. Chem. B 2006, 110, 14391–14397. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]



| P25 | Pt:TiO2(P25) | SiO2:TiO2(P25) | Pt:SiO2:TiO2(P25) | |
|---|---|---|---|---|
| Optimal Loading | – | 0.34 wt. % (Pt) | 1.2 wt. % (Si) | 0.6 wt. % (Pt) 1.9 wt. % (Si) |
| Kinetic Constant (min−1) | 0.044 | 0.169 | 0.065 | 0.267 |
| Improvement Ratio | 1 | 3.84 | 1.48 | 6.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benz, D.; Van Bui, H.; Hintzen, H.T.; Kreutzer, M.T.; van Ommen, J.R. Synthesis of a Rationally Designed Multi-Component Photocatalyst Pt:SiO2:TiO2(P25) with Improved Activity for Dye Degradation by Atomic Layer Deposition. Nanomaterials 2020, 10, 1496. https://doi.org/10.3390/nano10081496
Benz D, Van Bui H, Hintzen HT, Kreutzer MT, van Ommen JR. Synthesis of a Rationally Designed Multi-Component Photocatalyst Pt:SiO2:TiO2(P25) with Improved Activity for Dye Degradation by Atomic Layer Deposition. Nanomaterials. 2020; 10(8):1496. https://doi.org/10.3390/nano10081496
Chicago/Turabian StyleBenz, Dominik, Hao Van Bui, Hubertus T. Hintzen, Michiel T. Kreutzer, and J. Ruud van Ommen. 2020. "Synthesis of a Rationally Designed Multi-Component Photocatalyst Pt:SiO2:TiO2(P25) with Improved Activity for Dye Degradation by Atomic Layer Deposition" Nanomaterials 10, no. 8: 1496. https://doi.org/10.3390/nano10081496