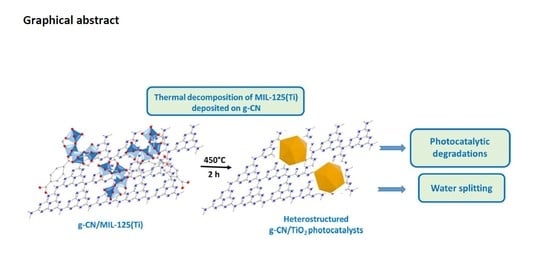
Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of MIL-125(Ti)
2.3. Synthesis of Bulk g-CN
2.4. Exfoliation of g-CN
2.5. Preparation of g-CN/TiO2 Photocatalysts
2.6. Photocatalytic Degradations
2.7. Photocatalytic H2 Production
2.8. Apparent Quantum Efficiency (AQE)
2.9. Photocatalyst Characterization
3. Results
3.1. Photocatalysts Synthesis and Characterization
3.2. Photodegradation of Pollutants under Visible Light Irradiation
3.3. Active Species Involved in the Photodegradation, Photoelectrochemical Measurements, and Mechanism
3.4. Hydrogen Photoproduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lou, X.W. Design of Heterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Mater. 2019, 31, 1900281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Shayegen, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrog. Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.P.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Shwetharani, R.; Sakar, M.; Fernando, C.A.N.; Binas, V.; Balakrishna, R.G. Recent advances and strategies to tailor the energy levels, active sites and electron mobility in titania and its doped/composite analogues for hydrogen evolution in sunlight. Catal. Sci. Technol. 2019, 9, 12–49. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Moussa, H.; Chouchene, B.; Gries, T.; Balan, L.; Mozet, K.; Medjahdi, G.; Schneider, R. Growth of ZnO Nanorods on Graphitic Carbon Nitride gCN Sheets for the Preparation of Photocatalysts with High Visible-Light Activity. ChemCatChem 2018, 10, 4973–4983. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Chouchene, B.; Desmarets, C.; Gries, T.; Balan, L.; Fournet, R.; Medjahdi, G.; Bayo, K.; Schneider, R. Copper octacarboxyphthalocyanine as sensitizer of graphitic carbon nitride for efficient dye degradation under visible light irradiation. Appl. Catal. A Gen. 2018, 563, 127–136. [Google Scholar] [CrossRef]
- Obregon, S.; Colon, G. Improved H2 production of Pt-TiO2/g-C3N4-MnOx composites by an efficient handling of photogenerated charge pairs. Appl. Catal. B Environ. 2014, 144, 775–782. [Google Scholar] [CrossRef]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 Mesocrystals Composite for H2 Evolution under Visible-Light Irradiation and Its Charge Carrier Dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef]
- Troppova, I.; Sihar, M.; Rehi, M.; Ritz, M.; Praus, P.; Koci, K. Unconventionally prepared TiO2/g-C3N4 photocatalysts for photocatalytic decomposition of nitrous oxide. Appl. Surf. Sci. 2018, 430, 335–347. [Google Scholar] [CrossRef]
- Sadanandam, G.; Zhang, L.; Scurrell, M.S. Enhanced photocatalytic hydrogen formation over Fe-loaded TiO2 and g-C3N4 composites from mixed glycerol and water by solar irradiation. J. Renew. Sustain. Energy 2018, 10, 034703. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhara, S.K.; Bellamkonda, S.; Rao, G.R.; Shankar, M.V.; Bahnemann, D.W.; Nappolian, B. Construction of ternary hybrid layered reduced graphene oxide supported g-C3N4-TiO2 nanocomposite and its photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2018, 43, 3892–3904. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.; Lei, Y.; Wang, B.; Wu, N.; Shi, Q.; Li, Q. In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H2 production and degradation. Nano Res. 2015, 8, 1199–1209. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs (1 0 1) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 hierarchical spheres with reactive {001} TiO2 crystal facets and its visible-light photocatalytic activity. Int. J. Hydrog. Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Pan, J.; You, M.; Chi, C.; Dong, Z.; Wang, B.; Zhu, M.; Zhao, W.; Song, C.; Zhang, Y.; Li, C. The two dimension carbon quantum dots modified porous g-C3N4/TiO2 nano-heterojunctions for visible light hydrogen production enhancement. Int. J. Hydrog. Energy 2018, 43, 6586–6593. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Giannakopoulo, T.; Papoilias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, X.; Li, Q.; Yang, J. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Appl. Catal. B Environ. 2017, 212, 106–114. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient Photoelectrochemical Water Splitting by g-C3N4/TiO2 Nanotube Array Heterostructures. Nano Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Sheng, Y.; Wai, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Li, C.; Lou, Z.; Yang, Y.; Wang, Y.; Lu, Y.; Ye, Z.; Zhu, L. Hollow sphere Nanoheterojunction of g-C3N4@TiO2 with High Visible Light Photocatalytic Property. Langmuir 2019, 35, 779–786. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 33, 183–192. [Google Scholar] [CrossRef]
- Zhan, W.; Sun, L.; Han, X. Recent progress on engineering highly efficient porous semiconductor photocatalysts derived from Metal–organic frameworks. Nano-Micro Lett. 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Guan, P.-Y.; Li, Q.-Y.; Zhang, M.; Zang, S.-Q. MOF-derived flower-like MoS2@TiO2 nanohybrids with enhanced activity for hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 26794–26800. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, K.; Wu, Y.-N.; Wang, Y.; Morlay, C.; Li, F. Metal–organic framework-templated synthesis of bifunctional N-doped TiO2–carbon nanotablets via solid-state thermolysis. ACS Sustain. Chem. Eng. 2016, 4, 6744–6753. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, L.; Tang, Z.; Al-Mamun, M.; Zhao, H.; Su, X. Palladium-decorated hierarchical titania constructed from the metal-organic frameworks NH2-MIL-125(Ti) as a robust photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2017, 218, 743–750. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Dou, J.; Li, Y.; Xie, F.; Ding, X.; Wei, M. Metal–Organic Framework Derived Hierarchical Porous Anatase TiO2 as a Photoanode for Dye-Sensitized Solar Cell. Cryst. Growth Des. 2016, 16, 121–125. [Google Scholar] [CrossRef]
- Xiu, Z.; Alfaruqi, M.H.; Gim, J.; Song, J.; Kim, S.; Thi, T.V.; Duong, P.T.; Baboo, J.P.; Mathew, V.; Kim, J. Hierarchical porous anatase TiO2 derived from a titanium metal–organic framework as a superior anode material for lithium ion batteries. Chem. Commun. 2015, 51, 12274–12277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Cheng, J.K.; Hu, Z.; Zhang, M.; Xu, Q.; Kang, Z.; Zhao, D. Metal-organic frameworks (MOFs) as precursors towards TiOx/C composites for photodegradation of organic dye. RSC Adv. 2014, 4, 34221–34225. [Google Scholar] [CrossRef] [Green Version]
- Khaletskaya, K.; Pougin, A.; Medishetty, R.; Rösler, C.; Wiktor, C.; Strunk, J.; Fischer, R.A. Fabrication of Gold/Titania Photocatalyst for CO2 Reduction Based on Pyrolytic Conversion of the Metal–Organic Framework NH2-MIL-125(Ti) Loaded with Gold Nanoparticles. Chem. Mater. 2015, 27, 7248–7257. [Google Scholar] [CrossRef]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Hydrothermal fabrication of few-layer MoS2 nanosheets within nanopores on TiO2 derived from MIL-125(Ti) for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2017, 426, 177–184. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Chen, J.; Li, Y.; Zhao, J.; Gao, K.; Na, P. Effective elimination of As(iii) via simultaneous photocatalytic oxidation and adsorption by a bifunctional cake-like TiO2 derived from MIL-125(Ti). Catal. Sci. Technol. 2018, 8, 1936–1944. [Google Scholar] [CrossRef]
- Yuan, S.; Gao, Y.; Bao, J.; Zhang, Y.; Chen, W.; Fang, J.; Zhou, Y.; Wang, Y.; Zhu, W. Preparation of disk-like Pt/CeO2-p-TiO2 catalyst derived from MIL-125(Ti) for excellent catalytic performance. Appl. Organomet. Chem. 2018, 32, e4395. [Google Scholar] [CrossRef]
- Gao, K.; Chen, J.; Liu, Z.; Li, Y.; Wu, Y.; Zhao, J.; Na, P. Intensified redox co-conversion of As(III) and Cr(VI) with MIL-125(Ti)-derived COOH functionalized TiO2: Performance and mechanism. Chem. Eng. J. 2019, 360, 1223–1232. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Santaclara, J.G.; Oar-Arteta, L.; van Koppen, L.; Osadchii, D.Y.; Gascon, J.; Kapteijn, F. Photocatalytic properties of TiO2 and Fe-doped TiO2 prepared by metal organic framework-mediated synthesis. Chem. Eng. J. 2019, 360, 75–88. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Li, K.; Zuo, Y.; Zhang, A.; Song, C.; Zhang, G.; Guo, X. Solvothermal synthesis of NH2-MIL-125(Ti) from circular plate to octahedron. CrystEngComm 2014, 16, 9645–9650. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Park, J.H.; Kim, J.-h.; Ahn, S.; Lee, C.S. Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (g-C3N4) with highly enhanced photocatalytic activity and stability. Sci. Rep. 2016, 6, 31147. [Google Scholar] [CrossRef]
- Music, S.; Gotic, M.; Ivanda, M.; Popovic, S.; Twkovic, A.; Trojko, R.; Sekulic, A.; Furic, K. Chemical and micro structural properties of TiO2 synthesized by sol-gel procedure. Mater. Sci. Eng. B 1997, 47, 33–40. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Raziq, F.; Qu, Y.; Humayun, M.; Zada, A.; Yu, H.; Jing, L. Synthesis of SnO2/B-P codoped g-C3N4 nanocomposites as efficient cocatalyst-free visible-light photocatalysts for CO2 conversion and pollutant degradation. Appl. Catal. B Environ. 2017, 201, 486–494. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Jia, F.; Xie, J.; Li, W. Three-Dimensional Phosphorus-Doped Graphitic-C3N4 Self-Assembly with NH2-Functionalized Carbon Composite Materials for Enhanced Oxygen Reduction Reaction. Langmuir 2016, 32, 12569–12578. [Google Scholar] [CrossRef]
- Sun, P.; Xu, R.; Zhang, W.; Zada, I.; Liu, Q.; Gu, J.; Su, H.; Zhang, Z.; Zhang, J.; Zhang, D. Photocatalyst of organic pollutants decomposition: TiO2/glass fiber cloth composites. Catal. Today 2016, 274, 2–7. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-doped Anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Mandzya, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Leong, S.; Li, D.; Hapgood, K.; Zhang, X.; Wang, H. Ni(OH)2 decorated rutile TiO2 for efficient removal of tetracycline from wastewater. Appl. Catal. B Environ. 2016, 198, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Cetocioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barcelo, D.; Orhon, D.; Ince, O. Chronic impact of tetracycline on the biodegradation of an organic substrate mixture under anaerobic conditions. Water. Res. 2013, 47, 2959–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, L.-C.; Liu, Y.-T.; Syu, C.-H.; Huang, M.-H.; Tzou, Y.-M.; Teah, H.Y. Adsorption of tetracycline on Fe (hydr)oxides: Effects pf pH and metal cation (Cu2+, Zn2+ and Al3+) addition in various molar ratios. R. Soc. Open sci. 2018, 5, 171941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Peer Mohamed, A.A.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, Bifunctional Granules of Carbon-Doped g-C3N4 as Adsorptive Photocatalyst for the Efficient Removal of Tetracycline Antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Wang, H.; Wu, Z.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Cheng, B.; Yu, J. Direct Z-scheme TiO2/NiS Core-Shell Hybrid Nanofibers with Enhanced Photocatalytic H2-Production Activity. ACS Sustain. Chem. Eng. 2018, 6, 12291–12298. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Su, J.; Shi, J.; Wang, X.; Guo, L. Photocatalytic hydrogen production using twinned nanocrystals and an unanchored NiSx co-catalyst. Nat. Energy 2016, 1, 16151. [Google Scholar] [CrossRef]











| Theoretical g-CN:TiO2 Ratio | 1:1 | 2:1 | 3:1 | 4:1 | 8:1 |
| Actual g-CN:TiO2 Ratio | 0.28:1 | 0.91:1 | 1.59:1 | 2.12:1 | 4.41:1 |
| Sample | BET (m2g−1) | Pore Volume (cm3g−1) | Pore Size (nm) |
|---|---|---|---|
| g-CN | 9.8 ± 0.2 | 0.07 | 17.75 |
| TiO2 | 56.3 ± 0.4 | 0.18 | 10.19 |
| g-CN/TiO2 (1:1) | 59.8 ± 0.3 | 0.20 | 11.15 |
| g-CN/TiO2 (2:1) | 102.2 ± 0.4 | 0.29 | 9.42 |
| g-CN/TiO2 (3:1) | 86.2 ± 0.3 | 0.23 | 9.53 |
| g-CN/TiO2 (4:1) | 63.8 ± 0.1 | 0.20 | 11.19 |
| g-CN/TiO2 (8:1) | 48.8 ± 0.2 | 0.21 | 16.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, E.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials 2020, 10, 1387. https://doi.org/10.3390/nano10071387
Tatykayev B, Chouchene B, Balan L, Gries T, Medjahdi G, Girot E, Uralbekov B, Schneider R. Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production. Nanomaterials. 2020; 10(7):1387. https://doi.org/10.3390/nano10071387
Chicago/Turabian StyleTatykayev, Batukhan, Bilel Chouchene, Lavinia Balan, Thomas Gries, Ghouti Medjahdi, Emilien Girot, Bolat Uralbekov, and Raphaël Schneider. 2020. "Heterostructured g-CN/TiO2 Photocatalysts Prepared by Thermolysis of g-CN/MIL-125(Ti) Composites for Efficient Pollutant Degradation and Hydrogen Production" Nanomaterials 10, no. 7: 1387. https://doi.org/10.3390/nano10071387