Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid Lipid Nanoparticles (SLN)
2.3. Photon Correlation Spectroscopy (PCS) Analysis
2.4. Cryogenic Transmission Electron Microscopy (Cryo-TEM) Analysis
2.5. X-ray Scattering
2.6. Evaluation of CA Loading into SLN
2.7. Gel Preparation
2.8. Rheological Analysis
2.9. Spreadability Studies
2.10. In Vitro Diffusion Experiments
2.11. HPLC Procedure
2.12. Human Skin Explant Culture
2.13. Cigarette Smoke Exposure
2.14. Immunohistochemistry
2.15. Protein Extraction
2.16. Western Blot Analysis
2.17. Statistical Analysis
3. Results
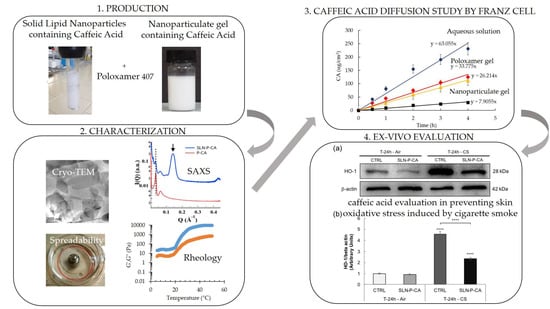
3.1. SLN Production and Characterization
3.1.1. Morphological Analysis
3.1.2. Dimensional Distribution
3.1.3. Evaluation of CA Encapsulation Efficiency in SLN
3.2. Preparation and Characterization of Gels
3.2.1. X-ray Scattering Analysis
3.2.2. Rheological Study
3.2.3. Gel Spreadability Study
3.2.4. In Vitro CA Diffusion Kinetics from Gels
3.3. Preparation and Characterization of Nanoparticulate Gels
3.3.1. X-Ray Scattering Analysis
3.3.2. Rheological and Spreadability Studies
3.3.3. In Vitro CA Diffusion Kinetics
3.4. Ex-Vivo Evaluation of SLN-P-CA in Protecting Human Skin against Oxidative Damage
3.4.1. Immunofluorescence Staining
3.4.2. Western Blot Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine 2004, 11, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Prasad, N.; Jeyanthimala, K.; Ramachandran, S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J. Photochem. Photobiol. B 2009, 95, 196–203. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.A.; Katyar, S.K. Skin photoprotection and natural polyphenol: Antiinflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Furrukh, M. Tobacco smoking and lung cancer: Perception-changing facts. Sultan Qaboos Univ. Med. J. 2013, 13, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Macacu, A.; Autier, P.; Boniol, M.; Boyle, P. Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 154, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical effects of cigarette smoking: Epidemiologic impact and review of pharmacotherapy option. Int. J. Environ. Res. Public Health 2017, 14, 1147. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, A.; Grando, S.A. Smoking and the skin. Int. J. Dermatol. 2012, 51, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Prieux, R.; Eeman, M.; Rothen-Rutishauser, B.; Valacchi, G. Mimicking cigarette smoke exposure to assess cutaneous toxicity. Toxicol. In Vitro 2020, 62, 104664–104675. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Han, M.-L.; Teng, N.-C.; Lee, C.-Y.; Huang, W.-T.; Lin, C.-T.; Huang, Y.-K. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis- an observational study. Sci. Rep. 2018, 8, 11055–11065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeur, J.; Belaïdi, J.P.; Chollet, C.; Denat, L.; Dimitrov, A.; Jones, C.; Perez, P.; Zanini, M.; Zobiri, O.; Mezzache, S.; et al. Photo-pollution stress in skin: Traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017, 86, 162–169. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid nanostructures for antioxidant delivery: A comparative preformulation study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef]
- Esposito, E.; Fantin, M.; Marti, M.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Sivieri, E.; Lain, F.; Menegatti, E.; Morari, M.; et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm. Res. 2008, 25, 1521–1530. [Google Scholar] [CrossRef]
- Pink, D.L.; Loruthai, O.; Ziolek, R.M.; Wasutrasawat, P.; Terry, A.E.M.; Lawrence, J.; Lorenz, C.D. On the structure of solid lipid nanoparticles. Nano Micro Small 2019, 15, 1903156. [Google Scholar] [CrossRef]
- Yadav, N.; Khatak, S.; Sara, U.V.S. Solid lipid nanoparticles- A review. Int. J. Appl. Pharm. 2013, 5, 8–18. [Google Scholar]
- Esposito, E.; Ravani, L.; Contado, C.; Costenaro, A.; Drechsler, M.; Rossi, D.; Menegatti, E.; Grandini, A.; Cortesi, R. Clotrimazole nanoparticle gel for mucosal administration. Mater. Sci. Eng. C 2013, 33, 411–418. [Google Scholar] [CrossRef]
- Artzner, F.; Geiger, S.; Olivier, S.; Allais, C.; Finet, S.; Agnely, F. Interactions between poloxamers in aqueous solutions: Micellization and gelation studied by differential scanning calorimetry, Small Angle X-ray Scattering, and Rheology. Langmuir 2007, 23, 5085–5092. [Google Scholar] [CrossRef]
- Garrastazu Pereira, G.; Anversa Dimer, F.; Stanisçuaki, S. Formulation and characterization of poloxamer 407®: Thermoreversible gel containing polymeric microparticles and hyaluronic acid. Quim. Nova 2013, 36, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, A.; Feng, F.; Jiang, Q.; Sun, H.; Chai, Y.; Yang, R.; Wang, Z.; Hou, J.; Li, R. Effect of the hyaluronic acid-poloxamer hydrogel on skin-wound healing: In vitro and in vivo studies. Anim. Model. Exp. Med. 2019, 2, 107–113. [Google Scholar] [CrossRef]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F.; Rizza, L.; Cortesi, R.; Merlotti, E.; Drechsler, M.; Mariani, P.; Contado, C.; Ravani, L.; Esposito, E. Evaluation of percutaneous absorption of naproxen from different liposomal formulations. J. Pharm. Sci. 2010, 99, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and characterization of a clotrimazole liposphere gel for candidiasis treatment. Polymers 2018, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Esposito, E.; Sticozzi, C.; Ravani, L.; Drechsler, M.; Muresan, X.M.; Cervellati, F.; Cortesi, R.; Valacchi, G. Effect of new curcumin-containing nanostructured lipid dispersions on human keratinocytes proliferative responses. Exp. Dermatol. 2015, 24, 449–454. [Google Scholar] [CrossRef]
- Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Savelli, V.; Evelson, P.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 78–85. [Google Scholar] [CrossRef]
- Pecorelli, A.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Hayek, J.; De Felice, C.; Valacchi, G. Increased levels of 4HNE-protein plasma adducts in Rett syndrome. Clin Biochem. 2011, 44, 368–371. [Google Scholar] [CrossRef]
- Kulkarni, C.V. Lipid crystallization: From self-assembly to hierarchical and biological ordering. Nanoscale 2012, 4, 5779–5791. [Google Scholar] [CrossRef] [PubMed]
- Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Ferrara, F.; Lila, M.A.; Valacchi, G. SR-B1 involvement in keratinocytes in vitro wound closure. Arch. Biochem. Biophys. 2018, 658, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Pecorelli, A.; Belmonte, G.; Pambianchi, E.; Cervellati, F.; Lynch, S.; Krol, Y.; Oresajo, C. Protective effects of topical vitamin c compound mixtures against ozone-induced damage in human skin. J. Investig. Dermatol. 2017, 137, 1373–1375. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Xu, H.; Ding, P.T.; Li, S.M.; Zheng, J.M. Thermosetting gels with modulated gelation temperature for ophthalmic use: The rheological and gamma scintigraphic studies. J. Control. Release 2002, 83, 65–74. [Google Scholar] [CrossRef]
- Matsuoka, H.; Tanaka, H.; Iizuka, N.; Hashimoto, T.; Ise, N. Elastic scattering from cubic lattice systems with paracrystalline distortion. II. Phys. Rev. B 1990, 41, 3854–3856. [Google Scholar] [CrossRef]
- Baloglu, E.; Yaprak, K.S.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Dumortier, G.; Kateb, N.; Sahli, M.; Kedjar, S.; Boulliat, A.; Chaumeil, J.-C. Development of a Thermogelling Ophthalmic Formulation of Cysteine. Drug Dev. Ind. Pharm. 2006, 32, 63–72. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomat. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging—Contributors and inhibitors. J. Cosm. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. BioFactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Sticozzi, C.; Cervellati, F.; Muresan, X.M.; Cervellati, C.; Valacchi, G. Resveratrol prevents cigarette smoke-induced keratinocytes damage. Food Funct. 2014, 5, 2348–2356. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Nanjappa, V.; Raja, R.; Jain, A.P.; Mangalaparthi, K.K.; Sathe, G.J.; Babu, N.; Patel, K.; Cavusoglu, N.; Soeur, J.; et al. How Does Chronic Cigarette Smoke Exposure Affect Human Skin? A Global Proteomics Study in Primary Human Keratinocytes. OMICS J. Integr. Biol. 2016, 20, 615–626. [Google Scholar] [CrossRef]
- Kubo, H.; Asai, K.; Kojima, K.; Sugitani, A.; Kyomoto, Y.; Okamoto, A.; Yamada, K.; Ijiri, N.; Watanabe, T.; Hirata, K.; et al. Astaxanthin suppresses cigarette smoke-induced emphysema through Nrf2 activation in mice. Mar. Drugs 2019, 17, 673. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Mirlohi, M.; Varshosaz, J.; Madani, G. Novel caffeic acid nanocarrier: Production, characterization, and release modeling. J. Nanomater. 2013, 2013, 434632. [Google Scholar] [CrossRef]
- Yang, S.; Pyo, M.C.; Nam, M.; Lee, K.W. ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement. Altern. Med. 2019, 139, 19. [Google Scholar] [CrossRef] [Green Version]










| Nanoparticulate System | Tristearin | p188 1 | Water | CA 2 |
|---|---|---|---|---|
| SLN | 5 | 2.38 | 92.62 | - |
| SLN-CA | 5 | 2.38 | 92.52 | 0.1 |
| Nanoparticulate System | Z-Average (nm) ± s.d. 1 | Dispersity Index ± s.d. 1 | Encapsulation Efficiency (%) 2 | Loading Capacity (%) 3 |
|---|---|---|---|---|
| SLN | 216 ± 12 | 0.28 ± 0.02 | - | - |
| SLN-CA SLN-P SLN-P-CA | 201 ± 11 225 ± 10 230 ± 14 | 0.29 ± 0.03 0.23 ± 0.04 0.26 ± 0.03 | 88.2 ± 8.3 - 87.8 ± 5.2 | 1.8 ± 0.03 - 1.7 ± 0.05 |
| Gel System | Tristearin | p188 1 | p407 2 HA | HA 3 HA | Water HA | CA 4 HA |
|---|---|---|---|---|---|---|
| P P-HA P-CA P-HA-CA SLN-P SLN-P-CA P-P188-CA | - - - - 4.25 4.25 - | - - - - 2.02 2.02 2.02 | 15.0 15.0 15.0 15.0 15.0 15.0 15.0 | - 2.0 - 2.0 - - - | 85.0 83.0 84.9 82.9 78.52 78.42 82.88 | - - 0.1 0.1 - 0.1 0.1 |
| Fitting Parameter | P (20 °C) | P (37 °C) | P-CA (37 °C) | P-HA-CA (37 °C) |
|---|---|---|---|---|
| D (nm) lattice distortion micelle radius (nm) | 24.8 0.18 7.9 | 24.7 0.14 9.4 | 25.0 0.11 10.5 | 26.3 0.12 12.1 |
| reduced chi-squared | 10.5 | 6.4 | 5.5 | 5.3 |
| Gel | Tsol-gel | Spreadability (g × cm/s) |
|---|---|---|
| P P-CA P-HA P-HA-CA P-P188-CA SLN-P | 25.1 ± 3.1 20.9 ± 2.1 22.0 ± 2.2 24.8 ± 5.2 28.1 ± 1.2 - | 11.41 ± 1.88 11.40 ± 1.75 12.50 ± 1.30 12.45 ± 1.20 - 10.02 ± 1.68 |
| SLN-P-CA | - | 10.00 ± 1.81 |
| Formulation | F 1 (µg/cm2/h) | D 2 (cm/h) |
|---|---|---|
| Sol-CA P-HA-CA P-CA SLN-CA SLN-P-CA | 63.05 ± 4.41 40.23 ± 2.81 33.77 ± 2.36 26.21 ± 1.83 7.90 ± 0.55 | 126.11 ± 8.82 40.23 ± 2.81 33.77 ± 2.36 26.21 ± 1.83 7.90 ± 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. https://doi.org/10.3390/nano10050961
Sguizzato M, Mariani P, Ferrara F, Drechsler M, Hallan SS, Huang N, Simelière F, Khunti N, Cortesi R, Marchetti N, et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials. 2020; 10(5):961. https://doi.org/10.3390/nano10050961
Chicago/Turabian StyleSguizzato, Maddalena, Paolo Mariani, Francesca Ferrara, Markus Drechsler, Supandeep Singh Hallan, Nicolas Huang, Fanny Simelière, Nikul Khunti, Rita Cortesi, Nicola Marchetti, and et al. 2020. "Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid" Nanomaterials 10, no. 5: 961. https://doi.org/10.3390/nano10050961