Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Catalysts
2.1.1. Synthesis of Bi2WO6
2.1.2. Synthesis of TiO2/C-Dots
2.1.3. Synthesis of Bi2WO6/C-Dots/TiO2
2.2. Characterizations of Prepared Catalysts
2.3. Photocatalytic Experiments
2.4. Investigation of Function of Active Radical Ions in the Decomposition of Levofloxacin under Sunlight Illumination
3. Results and Discussion
3.1. Characterization of the Synthesized Catalysts
3.2. Photocatalytic Activity of Z-Scheme Nano-Photocatalyst under Solar Light Illumination
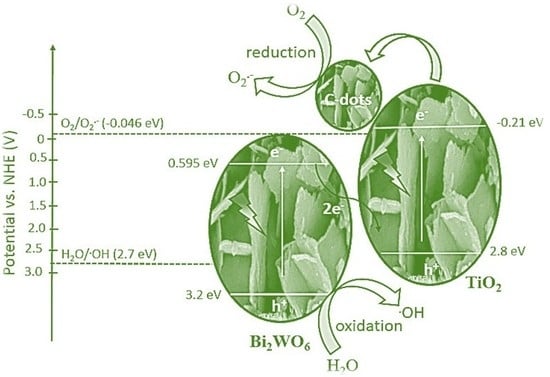
3.3. Plausible Mechanism for Levofloxacin Degradation with Z-Scheme Catalyst under Solar Light Irradiation
3.4. Investigation of Degradation Intermediates Formed in the Photocatalytic Degradation of Levofloxacin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorensen, B.H.; Nielsen, N.; Lanzky, P.F.; Ingerslev, F.; Holten Lutzhoft, H.C.; Jorgensen, S.E. Occurrence fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1988, 36, 357–393. [Google Scholar] [CrossRef]
- An, J.-B.; Hu, D.-P.; Li, Y.-L.; Chen, N.-L. Efficient Degradation of Atrazine by Magnetic CoFe2O4/g-C3N4 Catalyzed Peroxymonosulfate and Its Enhancement of Photocatalytic Ability under Visible-Light. Sci. Adv. Mater. 2019, 11, 1764–1772. [Google Scholar] [CrossRef]
- Abellan, M.N.; Gimenez, J.; Esplugas, S. Photocatalytic degradation of antibiotics: The case of sulfamethoxazole and trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Taneja, P.; Sharma, S.; Umar, A.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Visible-light driven photocatalytic degradation of brilliant green dye based on cobalt tungstate nanoparticles. Mater. Chem. Phys. 2018, 211, 335–342. [Google Scholar] [CrossRef]
- Mortazavi, S.; Khakpoor, A.A.; Mehrzad, A.; Siamy, B. Study of the Photocatalytic Activity and Hydrophilic Properties in the Titanium Dioxide Layer Deposited on Copper Nanoclusters. J. Nanoelectron. Optoelectron. 2019, 14, 272–279. [Google Scholar] [CrossRef]
- Hignite, C.; Azarnoff, D.L. Drugs and drug metabolites as environmental contaminants: Chlorophenoxyisobutyrate and salicyclic acid in sewage water effluent. Life Sci. 1977, 20, 337–341. [Google Scholar] [CrossRef]
- Qi, X.; Liu, P.; Yao, F.; Liu, J.; Vadivel, S. Facile Synthesis of Visible-Light-Active MoO3/Ag2CrO4 Heterojunction Photocatalyst with Remarkably Enhanced Photocatalytic Activity Towards Tartrazine. Sci. Adv. Mater. 2019, 11, 1162–1167. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. Photocatalytic degradation and decomposition mechanism of fluoroquinolones norfloxacin over bismuth tungstate: Experiment and mathematic model. Appl. Catal. B 2015, 168–169, 175–182. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Qiu, L.; Wang, Y.; Xiao, L.; Ouyang, F.; Lin, L.; Chen, X. Effect of Silver Doping on F-TiO2/SiO2 Nano-Powder Catalysts for Photocatalytic Degradation of Acrylonitrile Wastewater. J. Nanoelectron. Optoelectron. 2019, 14, 1043–1047. [Google Scholar] [CrossRef]
- Najjar, N.H.E.; Touffet, A.; Deborde, M.; Journal, R.; Leitner, N.K.V. Levofloxacin oxidation by ozone and hydroxyl radicals: Kinetic study, transformation products and toxicity. Chemosphere 2013, 93, 604–611. [Google Scholar] [CrossRef]
- Cao, Z.; Li, W.; Pang, J.; Xu, J.; Jiao, Y.; Li, X. Mesoporous TiO2 with Tunable Mixed Phase (Anatase/Rutile) and Morphology by Using Polyethyleneimine Modified F127 Block Copolymers as Templates for Enhanced Photocatalytic Performance. Sci. Adv. Mater. 2019, 11, 166–177. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018, 360, 34–43. [Google Scholar] [CrossRef]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ionactivated persulfate and combined Fenton/persulfate systems. Chem. Eng. J. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Abbas, N.; Abbas, T.; Ahmad, T.; Sajid, I.H.; Khalid, H.R.; Naqvi, S.R.; Shahzad, F. Inexpensive Sol Gel Synthesis of Highly Active and Environmentally Benign Expanded Graphite/TiO2 Hybrid Photocatalysts. J. Nanoelectron. Optoelectron. 2019, 14, 1482–1490. [Google Scholar] [CrossRef]
- Ge, L.; Na, G.; Zhang, S.; Li, K.; Zhang, P.; Ren, H.; Yao, Z. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes. Sci. Total Environ. 2015, 527–528, 12–17. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. Degradation of antibiotic norfloxacin in aqueous solution by visible-light-mediated C-TiO2 photocatalysis. J. Hazard. Mater. 2012, 219–220, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Oberle, K.; Capdeville, M.J.; Berthe, T.; Budzinski, H.; Petit, F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli; from medical center patients to a receiving environment. Environ. Sci. Technol. 2012, 46, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mehta, S.K.; Kansal, S.K. N doped ZnO/C-dots nanoflowers as visible light driven photocatalyst for the degradation of malachite green dye in aqueous phase. J. Alloys Compd. 2017, 699, 323–333. [Google Scholar] [CrossRef]
- Huang, D.; Long, Y.; Luo, L.; Li, L.; Zhang, S.; Wang, L.; Jiang, F. Synthesis of N-Doped Bi2O3 and Its Excellent Visible Light Photocatalytic Performance for the Degradation of 17β-Estradiol. Sci. Adv. Mater. 2019, 11, 105–111. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Sunlight-driven photocatalytic degradation of non-steroidal anti-inflammatory drug based on TiO2 quantum dots. J. Colloid Interface Sci. 2015, 459, 257–263. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Umar, A.; Jha, M.; Mehta, S.K.; Kansal, S.K. Nanocuboidal-shaped zirconium based metal organic framework (UiO-66) for the enhanced adsorptive removal of nonsteroidal anti-inflammatory drug, ketorolac tromethamine, from aqueous phase. New J. Chem. 2018, 42, 1921–1930. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Synergetic Effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, S.; Sood, S.; Umar, A.; Kansal, S.K.; Sulphide, B. (Bi2S3) nanotubes decorated TiO2 nanoparticles heterojunction assembly for enhanced solar light driven photocatalytic activity. Ceram. Int. 2016, 42, 17551–17557. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217. [Google Scholar] [CrossRef]
- Formal, F.L.; Pendlebury, S.R.; Cornuz, M.; Tilley, S.D.; Grä tzel, M.; Durrant, J.R. Back electron–hole recombination in hematite photoanodes for water splitting. J. Am. Chem. Soc. 2014, 136, 2564. [Google Scholar] [CrossRef]
- Li, X.H.; Antonietti, M. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: Functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 2013, 42, 6593. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Physics and chemistry of CdTe/CdS thin film heterojunction photovoltaic devices: Fundamental and critical aspects. Energy Environ. Sci. 2014, 7, 45. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. A 2002, 148, 71. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3−/I− shuttle redox mediator under visible light irradiation. Chem. Commun. 2001, 23, 2416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Umar, A.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Solar light driven photocatalytic degradation of levofloxacin using TiO2/carbon-dot nanocomposites. New J. Chem. 2018, 42, 7445. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Y.; Xu, Z. One-step uniformly hybrid carbon quantum dots with high-reactive TiO2 for photocatalytic application. J. Alloys Compd. 2015, 622, 303–308. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Duan, L.; Shen, H.; Zhang, Y.; Zhao, X. In situ synthesis and enhanced visible light photocatalytic activity of C-TiO2 microspheres/carbon quantum dots. Ceram. Int. 2017, 43, 8648–8654. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Wu, Q.S.; Feng, Y.; Zhang, G.Y.; Sun, Y.Q.; Xu, Y.Y.; Gao, D.Z. a-Fe2O3 modified Bi2WO6 flower-like mesostructures with enhanced photocatalytic performance. Mater. Res. Bull. 2014, 49, 440–447. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Xiao, G.; Zhong, S.; Lu, C. Synthesis of Bi2WO6/Bi2O3 composite with enhanced photocatalytic activity by a facile one-step hydrothermal synthesis route. Photochem. Photobiol. 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Kumar, V.; Prasad, M.D.; Vithal, M. Enhanced visible light photocatalytic activity of Sn doped Bi2WO6 nanocrystals. Mater. Lett. 2015, 152, 200–202. [Google Scholar] [CrossRef]
- Xia, J.; Li, H.; Luo, Z.; Xu, H.; Wang, K.; Yin, S.; Yan, Y. Self-assembly and enhanced optical absorption of Bi2WO6 nest via ionic liquid-assisted hydrothermal method. Mater. Chem. Phys. 2010, 121, 6–9. [Google Scholar] [CrossRef]
- Cao, R.; Huang, H.; Tian, N.; Zhang, Y.; Guo, Y.; Zhang, T. Noval Y doped Bi2WO6 photocatalyst: Hydrothermal fabrication, characterization and enhanced visible-light-driven photocatalytic activity for rhodamine B degradation and photocurrent generation. Mater. Charact. 2015, 101, 166–172. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis, characterization and visible light-driven photocatalytic properties of Bi2WO6 nanoplates. J. Nanomater. 2014, 138561. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bai, X.; Pan, C.; He, J.; Zhu, Y. Enhancement of photocatalytic activity of Bi2WO6 hybridized with graphite-like C3N4. J. Mater. Chem. 2012, 22, 11568–11573. [Google Scholar] [CrossRef]
- Min, Y.; Zhang, K.; Chen, Y.; Zhang, Y.; Zhao, W. Synthesis of nanostructured ZnO/Bi2WO6 heterojunction for photocatalysis application. Sep. Purif. Technol. 2012, 92, 115–120. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, J.; Xiao, C.; Tan, X. Photocatalytic degradation of methylene blue over Co3O4/Bi2WO6 composite under visible light irradiation. Catal. Commun. 2008, 9, 1247–1253. [Google Scholar] [CrossRef]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Xiang, L.; Chen, L.; Mo, C.; Zheng, L.M.; Yu, Z.X.; Li, Y.W.; Cai, Q.Y.; Li, H.; Yang, W.D.; Zhou, D.M.; et al. Facile synthesis of Ni-doping Bi2WO6 nano-sheets with enhanced adsorptive and visible-light photocatalytic performances. J. Mater. Sci. 2018, 53, 7657–7671. [Google Scholar]
- Chou, J.C.; Liao, L.P. Study on pH at the point of zero charge of TiO2 pH ion-sensitive field effect transistor made by the sputtering method. Thin Solid Films 2005, 476, 157–161. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.L.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Kaur, A.; Salunke, D.B.; Umar, A.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Visible light driven photocatalytic degradation of fluoroquinolone levofloxacin drug using Ag2O/TiO2 quantum dots: A mechanistic study and degradation pathway. New J. Chem. 2017, 41, 12079–12090. [Google Scholar] [CrossRef]
- Hapeshi, E.; Fotiou, I.; Fatta-Kassinos, D. Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chem. Eng. J. 2013, 224, 96–105. [Google Scholar] [CrossRef]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Q.; Hou, J.; Yan, J.; Zhang, F.; Zhao, J.; Ding, H.; Li, Y.; Ding, L. One-step solvothermal synthesis of magnetic Fe3O4-graphite composite for Fenton-like degradation of levofloxacin. J. Environ. Sci. Health Part A 2016, 51, 52–62. [Google Scholar] [CrossRef] [PubMed]










| Element | Series | Unn. C [wt.%] | Norm. C [wt.%] | Atom. C [wt.%] | Error (3 Sigma) [wt.%] |
|---|---|---|---|---|---|
| Bismuth | M-Series | 17.10 | 14.74 | 5.10 | 1.98 |
| Carbon | K-Series | 6.61 | 5.70 | 34.33 | 4.22 |
| Oxygen | K-Series | 1.45 | 1.25 | 5.65 | 1.47 |
| Tungsten | L-Series | 65.80 | 56.73 | 22.32 | 7.86 |
| Titanium | K-Series | 25.03 | 21.58 | 32.60 | 2.37 |
| Total | 115.99 | 100.00 | 100.00 |
| S. No. | Photocatalyst | Rate Constant (k) s−1 | R2 |
|---|---|---|---|
| 1 | Bi2WO6/C-dots/TiO2 | 0.0007765 | 0.98797 |
| 2 | Bi2WO6 | 0.0004808 | 0.97777 |
| 3 | TiO2/C-dots | 0.000443 | 0.95016 |
| 4 | TiO2 | 0.00025183 | 0.96788 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Ibhadon, A.O.; Francesconi, M.G.; Mehta, S.K.; Elumalai, S.; Kansal, S.K.; Umar, A.; Baskoutas, S. Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium. Nanomaterials 2020, 10, 910. https://doi.org/10.3390/nano10050910
Sharma S, Ibhadon AO, Francesconi MG, Mehta SK, Elumalai S, Kansal SK, Umar A, Baskoutas S. Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium. Nanomaterials. 2020; 10(5):910. https://doi.org/10.3390/nano10050910
Chicago/Turabian StyleSharma, Shelja, Alex O. Ibhadon, M. Grazia Francesconi, Surinder Kumar Mehta, Sasikumar Elumalai, Sushil Kumar Kansal, Ahmad Umar, and Sotirios Baskoutas. 2020. "Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium" Nanomaterials 10, no. 5: 910. https://doi.org/10.3390/nano10050910