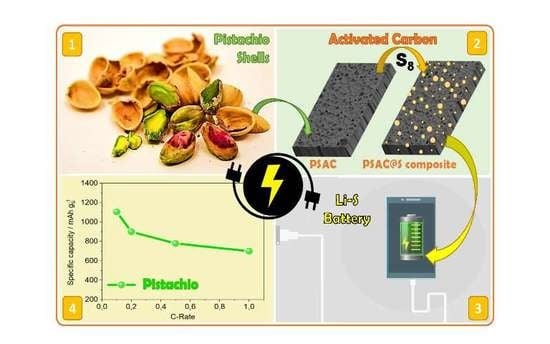
Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Electrode Preparation
2.2. Material Characterization
2.3. Cell Assembly and Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How far away are lithium-sulfur batteries from commercialization? Front. Energy Res. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Xu, J.; Ma, J.; Fan, Q.; Guo, S.; Dou, S. Recent progress in the design of advanced cathode materials and battery models for high-performance lithium-X (X = O2, S, Se, Te, I2, Br2) batteries. Adv. Mater. 2017, 29, 1606454. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-C.; Pan, R.; Lee, M.-T.; Nyholm, L.; Brandell, D.; Lacey, M.J. Cellulose separators with integrated carbon nanotube interlayers for lithium-sulfur batteries: An investigation into the complex interplay between cell components. J. Electrochem. Soc. 2019, 166, A3235–A3241. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.; Xue, L.; Jiao, Y.; Lei, T.; Chu, J.; Huang, J.; Gong, C.; Yan, C.; Yan, Y.; et al. Adsorption-catalysis design in the lithium-sulfur battery. Adv. Energy Mater. 2020, 10, 1903008. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. Long-life, high-rate lithium–sulfur cells with a carbon-free VN host as an efficient polysulfide adsorbent and lithium dendrite inhibitor. Adv. Energy Mater. 2020, 10, 1903241. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, S.; Chen, Y.; Cai, J.; Li, J.; Yang, Q.; Sun, J.; Liu, Z. enhanced sulfur redox and polysulfide regulation via porous VN-modified separator for Li–S batteries. ACS Appl. Mater. Interfaces 2019, 11, 5687–5694. [Google Scholar] [CrossRef]
- Fang, D.; Wang, Y.; Liu, X.; Yu, J.; Qian, C.; Chen, S.; Wang, X.; Zhang, S. Spider-web-inspired nanocomposite-modified separator: Structural and chemical cooperativity inhibiting the shuttle effect in Li-S batteries. ACS Nano 2019, 13, 1563–1573. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.; Xiong, X.; Han, K. Ultra-lightweight Ti3C2T MXene modified separator for Li–S batteries: Thickness regulation enabled polysulfide inhibition and lithium ion transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Wu, X.; Peng, Z.; Wang, J.; Gan, S.; Zhang, Y.; Han, D.; Niu, L. Compactly coupled nitrogen-doped carbon nanosheets/molybdenum phosphide nanocrystal hollow nanospheres as polysulfide reservoirs for high-performance lithium–sulfur chemistry. Small 2019, 15, 1902491. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Chen, L.; Lu, Y.; Su, Y.; Jia, Y.; Bao, L.; Wang, J.; Chen, S.; Chen, R. Metal-organic frameworks composites threaded on the CNT knitted separator for suppressing the shuttle effect of lithium sulfur batteries. Energy Storage Mater. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Paolella, A.; Demers, H.; Chevallier, P.; Gagnon, C.; Girard, G.; Delaporte, N.; Zhu, W.; Vijh, A.; Guerfi, A.; Zaghib, K. A platinum nanolayer on lithium metal as an interfacial barrier to shuttle effect in Li-S batteries. J. Power Sources 2019, 427, 201–206. [Google Scholar] [CrossRef]
- Paolella, A.; Laul, D.; Timoshevskii, V.; Zhu, W.; Marras, S.; Bertoni, G.; Wahba, A.S.; Girard, G.; Gagnon, C.; Rodrigue, L.; et al. The role of metal disulfide interlayer in Li–S batteries. J. Phys. Chem. C 2018, 122, 1014–1023. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.-J.; Li, B.-Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.-Q.; Zhang, Q. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium-sulfur batteries. Adv. Energy Mater. 2019, 9, 1802768. [Google Scholar] [CrossRef]
- Marceau, H.; Kim, C.-S.; Paolella, A.; Ladouceur, S.; Lagacé, M.; Chaker, M.; Vijh, A.; Guerfi, A.; Julien, C.M.; Mauger, A.; et al. In operando scanning electron microscopy and ultraviolet–visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte. J. Power Sources 2016, 319, 247–254. [Google Scholar] [CrossRef]
- Patel, M.D.; Cha, E.; Kang, C.; Gwalani, B.; Choi, W. High performance rechargeable Li-S batteries using binder-free large sulfur-loaded three-dimensional carbon nanotubes. Carbon 2017, 118, 120–126. [Google Scholar] [CrossRef]
- Zeng, L.; Pan, F.; Li, W.; Jiang, Y.; Zhong, X.; Yu, Y. Free-standing porous carbon nanofibers–sulfur composite for flexible Li–S battery cathode. Nanoscale 2014, 6, 9579–9587. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Shang, C.; Pan, X.; Chen, Z.; Shui, L.; Wang, X.; Zhou, G. Lotus root-like nitrogen-doped carbon nanofiber structure assembled with VN catalysts as a multifunctional host for superior lithium–sulfur batteries. Nanomaterials 2019, 9, 1724. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Han, D.; Wang, L.; Li, G.; Liu, S.; Gao, X. NiCo2O4 nanofibers as carbon-free sulfur immobilizer to fabricate sulfur-based composite with high volumetric capacity for lithium–sulfur battery. Adv. Energy Mater. 2019, 9, 1803477. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Morales, J.; Agostini, M.; Hassoun, J. Lithium battery using sulfur infiltrated in three-dimensional flower-like hierarchical porous carbon electrode. Mater. Chem. Phys. 2016, 180, 82–88. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Liu, X.-F.; Zhang, Q.; Tian, G.-L.; Huang, J.-Q.; Zhu, W.; Wei, F. Graphene/single-walled carbon nanotube hybrids: One-step catalytic growth and applications for high-rate Li–S batteries. ACS Nano 2012, 6, 10759–10769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Three-dimensional hierarchical porous structure of PPy/porous-graphene to encapsulate polysulfides for lithium/sulfur batteries. Nanomaterials 2018, 8, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Sun, L.; Wang, Z.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Three-dimensionally hierarchical graphene based aerogel encapsulated sulfur as cathode for lithium/sulfur batteries. Nanomaterials 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K.; Zhou, X.; Liu, Z. Graphene/sulfur/carbon nanocomposite for high performance lithium-sulfur batteries. Nanomaterials 2015, 5, 1481–1492. [Google Scholar] [CrossRef]
- Benítez, A.; Di Lecce, D.; Elia, G.A.; Caballero, Á.; Morales, J.; Hassoun, J. A lithium-ion battery using a 3 D-array nanostructured graphene–sulfur cathode and a silicon oxide-based anode. ChemSusChem 2018, 11, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Benítez, A.; Di Lecce, D.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E.; Hassoun, J. Lithium sulfur battery exploiting material design and electrolyte chemistry: 3D graphene framework and diglyme solution. J. Power Sources 2018, 397, 102–112. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Z.; Zhang, Y.; Tan, T.; Tian, Y.; Chen, Z. Novel sulfur/ethylenediamine-functionalized reduced graphene oxide composite as cathode material for high-performance lithium-sulfur batteries. Nanomaterials 2018, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Sun, Z.; Zhang, Y.; Wang, X.; Bakenov, Z.; Yin, F. Micro-spherical sulfur/graphene oxide composite via spray drying for high performance lithium sulfur batteries. Nanomaterials 2018, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Moreno, N.; Agostini, M.; Caballero, A.; Morales, J.; Hassoun, J. A long-life lithium ion sulfur battery exploiting high performance electrodes. Chem. Commun. 2015, 51, 14540–14542. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Hernán, L.; Morales, J.; Canales-Vázquez, J. Ordered mesoporous carbons obtained by a simple soft template method as sulfur immobilizers for lithium–sulfur cells. Phys. Chem. Chem. Phys. 2014, 16, 17332–17340. [Google Scholar] [CrossRef] [PubMed]
- Tesio, A.Y.; Arias, A.N.; Morales, J.; Planes, G.A.; Caballero, A. Versatility of a Nitrogen-containing monolithic porous carbon for lithium-based energy storage. ChemistrySelect 2018, 3, 8560–8567. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Luo, L.; Chen, Y.; Manthiram, A. Yolk-Shelled C@Fe3O4 Nanoboxes as efficient sulfur hosts for high-performance lithium-sulfur batteries. Adv. Mater. 2017, 29, 1702707. [Google Scholar] [CrossRef]
- Díez, N.; Ferrero, G.A.; Sevilla, M.; Fuertes, A.B. A simple and general approach for in situ synthesis of sulfur–porous carbon composites for lithium–sulfur batteries. Sustain. Energy Fuels 2019, 3, 3498–3509. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedel, C. Sustainable battery materials from biomass. ChemSusChem 2020, 92, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, Y.; Liang, C.; Yang, J.; Wang, J.; Nuli, Y. Confining small sulfur molecules in peanut shell-derived microporous graphitic carbon for advanced lithium sulfur battery. Electrochim. Acta 2018, 273, 127–135. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, M.; Wei, Y.; Zhao, C.; Carnie, M.; Yang, R.; Xu, Y. Process optimization for producing hierarchical porous bamboo-derived carbon materials with ultrahigh specific surface area for lithium-sulfur batteries. J. Alloys Compd. 2018, 738, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-H.; Du, X.-L.; He, J.-B.; Li, F.; Wang, Y.; Li, Y.-L.; Li, B.; Xin, S. Porous coconut shell carbon offering high retention and deep lithiation of sulfur for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 33855–33862. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Wang, C.; Huang, Z.; Hu, L.; Ke, X.; Liu, L.; Shi, Z.; Guo, Z. Walnut shell – Derived activated carbon: Synthesis and its application in the sulfur cathode for lithium–sulfur batteries. J. Alloys Compd. 2017, 718, 373–378. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Huang, L.; Maximov, M.; Jin, M.; Zhang, Y.; Wang, X.; Zhou, G. Biomass-derived oxygen and nitrogen Co-doped porous carbon with hierarchical architecture as sulfur hosts for high-performance lithium/sulfur batteries. Nanomaterials 2017, 7, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, N.; Caballero, A.; Hernán, L.; Morales, J. Lithium–sulfur batteries with activated carbons derived from olive stones. Carbon 2014, 70, 241–248. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, Á.; Morales, J.; Rodríguez-Castellón, E. Improved performance of electrodes based on carbonized olive stones/S composites by impregnating with mesoporous TiO2 for advanced Li—S batteries. J. Power Sources 2016, 313, 21–29. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Hernández-Rentero, C.; Caballero, A.; Morales, J. Biomass-derived carbon/γ-MnO2 nanorods/S composites prepared by facile procedures with improved performance for Li/S batteries. Electrochim. Acta 2018, 292, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Rentero, C.; Córdoba, R.; Moreno, N.; Caballero, A.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Low-cost disordered carbons for Li/S batteries: A high-performance carbon with dual porosity derived from cherry pits. Nano Res. 2018, 11, 89–100. [Google Scholar] [CrossRef]
- Benítez, A.; González-Tejero, M.; Caballero, Á.; Morales, J. Almond shell as a microporous carbon source for sustainable cathodes in lithium-sulfur batteries. Materials 2018, 11, 1428. [Google Scholar] [CrossRef] [Green Version]
- Özbek, H.N.; Fockink, D.H.; Yanık, D.K.; Göğüş, F.; Łukasik, R.M. The green biorefinery concept for the valorisation of pistachio shell by high-pressure CO2/H2O system. J. Clean. Prod. 2018, 196, 842–851. [Google Scholar] [CrossRef] [Green Version]
- Scholten, J.M.; Spanjer, M.C. Determination of Aflatoxin Bi in pistachio kernels and shells. J. AOAC Int. 1996, 79, 1360–1364. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Xia, P.; Lei, W.; Pan, Y.; Zou, Y.; Ma, Z. Preparation of activated carbon derived from biomass and its application in lithium–sulfur batteries. J. Porous Mater. 2019, 26, 1325–1333. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Improving the performance of biomass-derived carbons in Li-ion batteries by controlling the lithium insertion process. J. Electrochem. Soc. 2010, 157, A791. [Google Scholar] [CrossRef]
- Srinivasakannan, C. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar]
- Hulicova-Jurcakova, D.; Puziy, A.M.; Poddubnaya, O.I.; Suárez-García, F.; Tascón, J.M.D.; Lu, G.Q. Highly stable performance of supercapacitors from phosphorus-enriched carbons. J. Am. Chem. Soc. 2009, 131, 5026–5027. [Google Scholar] [CrossRef] [PubMed]
- Benítez, A.; Caballero, Á.; Rodríguez-Castellón, E.; Morales, J.; Hassoun, J. The role of current collector in enabling the high performance of Li/S battery. ChemistrySelect 2018, 3, 10371–10377. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Marangon, V.; Olivares-Marín, M.; Gómez-Serrano, V.; Caballero, Á.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J. Colloid Interface Sci. 2020, 573, 396–408. [Google Scholar] [CrossRef]
- Soler-Piña, F.J.; Hernández-Rentero, C.; Caballero, A.; Morales, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Highly graphitized carbon nanosheets with embedded Ni nanocrystals as anode for Li-ion batteries. Nano Res. 2020, 13, 86–94. [Google Scholar] [CrossRef]
- Barbosa, L.; Luna-Lama, F.; González Peña, Y.; Caballero, A. Simple and eco-friendly fabrication of electrode materials and their performance in high-voltage lithium-ion batteries. ChemSusChem 2020, 13, 838–849. [Google Scholar] [CrossRef]
- Seehra, M.S.; Pavlovic, A.S. X-Ray diffraction, thermal expansion, electrical conductivity, and optical microscopy studies of coal-based graphites. Carbon 1993, 31, 557–564. [Google Scholar] [CrossRef]
- Wang, S.; Zou, K.; Qian, Y.; Deng, Y.; Zhang, L.; Chen, G. Insight to the synergistic effect of N-doping level and pore structure on improving the electrochemical performance of sulfur/N-doped porous carbon cathode for Li-S batteries. Carbon 2019, 144, 745–755. [Google Scholar] [CrossRef]
- Zhu, Q.; Deng, H.; Su, Q.; Du, G.; Yu, Y.; Ma, S.; Xu, B. A free-standing nitrogen-doped porous carbon foam electrode derived from melaleuca bark for lithium-sulfur batteries. Electrochim. Acta 2019, 293, 19–24. [Google Scholar] [CrossRef]
- Hu, L.; Lu, Y.; Li, X.; Liang, J.; Huang, T.; Zhu, Y.; Qian, Y. Optimization of microporous carbon structures for lithium-sulfur battery applications in carbonate-based electrolyte. Small 2017, 13, 1603533. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Chen, K.; Ji, S.; Zhou, Y.; Wan, Y.; Xue, D.; Hodgson, P.; Li, Y. Facile synthesis of transition-metal oxide nanocrystals embedded in hollow carbon microspheres for high-rate lithium-ion-battery anodes. Chem. Eur. J. 2013, 19, 9811–9816. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Lai, C.; Liu, F.; Yang, W.; Hou, Y.; Zhang, S. A conductive interwoven bamboo carbon fiber membrane for Li–S batteries. J. Mater. Chem. A 2015, 3, 9502–9509. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhu, J.; Tiwary, C.S.; Ma, Z.; Huang, H.; Zhang, J.; Lu, Z.; Huang, W.; Wu, Y. Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 192, 99–109. [Google Scholar] [CrossRef]
- Baumann, A.E.; Aversa, G.E.; Roy, A.; Falk, M.L.; Bedford, N.M.; Thoi, V.S. Promoting sulfur adsorption using surface Cu sites in metal–organic frameworks for lithium sulfur batteries. J. Mater. Chem. A 2018, 6, 4811–4821. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.-J.; Jung, H.-G.; Sun, Y.-K.; Hassoun, J.; Scrosati, B. An advanced lithium-sulfur battery. Adv. Funct. Mater. 2013, 23, 1076–1080. [Google Scholar] [CrossRef]
- Zhu, W.; Paolella, A.; Kim, C.-S.; Liu, D.; Feng, Z.; Gagnon, C.; Trottier, J.; Vijh, A.; Guerfi, A.; Mauger, A.; et al. Investigation of the reaction mechanism of lithium sulfur batteries in different electrolyte systems by in situ Raman spectroscopy and in situ X-ray diffraction. Sustain. Energy Fuels 2017, 1, 737–747. [Google Scholar] [CrossRef]
- Paolella, A.; Zhu, W.; Marceau, H.; Kim, C.; Feng, Z.; Liu, D.; Gagnon, C.; Trottier, J.; Abdelbast, G.; Hovington, P.; et al. Transient existence of crystalline lithium disulfide Li2S2 in a lithium-sulfur battery. J. Power Sources 2016, 325, 641–645. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.; Paolella, A.; Gagnon, C.; Gariépy, V.; Vijh, A.; Zaghib, K. Application of operando X-ray diffraction and Raman spectroscopies in elucidating the behavior of cathode in lithium-ion batteries. Front. Energy Res. 2018, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Zeng, W.; Yin, Y.-X.; Zhang, J.; Yang, C.-P.; Zhu, Y.; Guo, Y.-G. Hierarchically micro/mesoporous activated graphene with a large surface area for high sulfur loading in Li–S batteries. J. Mater. Chem. A 2015, 3, 4799–4802. [Google Scholar] [CrossRef]
- Carbone, L.; Coneglian, T.; Gobet, M.; Munoz, S.; Devany, M.; Greenbaum, S.; Hassoun, J. A simple approach for making a viable, safe, and high-performances lithium-sulfur battery. J. Power Sources 2018, 377, 26–35. [Google Scholar] [CrossRef]
- Lv, Y.; Shang, M.; Chen, X.; Niu, J. Double-net enclosed sulfur composite as a new cathode in lithium sulfur batteries. J. Phys. Chem. C 2019, 123, 17719–17727. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Ahn, H.; Kim, O.; Park, M.J. Synthesis of three-dimensionally interconnected sulfur-rich polymers for cathode materials of high-rate lithium–sulfur batteries. Nat. Commun. 2015, 6, 7278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez, A.; Caballero, A.; Morales, J.; Hassoun, J.; Rodríguez-Castellón, E.; Canales-Vázquez, J. Physical activation of graphene: An effective, simple and clean procedure for obtaining microporous graphene for high-performance Li/S batteries. Nano Res. 2019, 12, 759–766. [Google Scholar] [CrossRef]
- Di Lecce, D.; Marangon, V.; Benitez, A.; Caballero, A.; Morales, J.; Rodríguez-Castellón, E.; Hassoun, J. High capacity semi-liquid lithium sulfur cells with enhanced reversibility for application in new-generation energy storage systems. J. Power Sources 2019, 412, 575–585. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Li, S.; Long, P.; Cao, C.; Cao, Y.; Wang, W.; Feng, Y.; Feng, W. A low cost ultra-microporous carbon scaffold with confined chain-like sulfur molecules as a superior cathode for lithium–sulfur batteries. Sustain. Energy Fuels 2018, 2, 2187–2196. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.H.; Zhang, Z.; Chen, Y.; Xiang, Y.; Liu, X.; Chen, J.S.; Chen, P. Naturally derived honeycomb-like N,S-codoped hierarchical porous carbon with MS2 (M = Co, Ni) decoration for high-performance Li–S battery. Nanoscale 2020, 12, 5114–5124. [Google Scholar] [CrossRef]






| Preparation Method | SBET m2·g−1 | S Loading mg·cm−2 | Long Term Cycling | Rate Capability | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Voltage Window V | Ci | Cf | Cycle | Rate | Rate | Caverage mAh·g−1 | ||||
| mAh·g−1 | ||||||||||
| Activation with ZnCl2, 550 °C S impregnation by melt diffusion (70:20:10) 1 | 1149 | 0.84 | 3.0–1.5 2 | 980 | 380 | 200 | C/5 | C/5 C/2 1C | 580 450 320 | [24] |
| Activation with H3PO4, 800 °C S impregnation by wet milling(80:10:10) 1 | 1345 | 2.5 | 2.6–1.8 | 1193 | 570 | 300 | C/10 | C/5 C/2 1C | 888 753 646 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, A.; Morales, J.; Caballero, Á. Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries. Nanomaterials 2020, 10, 840. https://doi.org/10.3390/nano10050840
Benítez A, Morales J, Caballero Á. Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries. Nanomaterials. 2020; 10(5):840. https://doi.org/10.3390/nano10050840
Chicago/Turabian StyleBenítez, Almudena, Julián Morales, and Álvaro Caballero. 2020. "Pistachio Shell-Derived Carbon Activated with Phosphoric Acid: A More Efficient Procedure to Improve the Performance of Li–S Batteries" Nanomaterials 10, no. 5: 840. https://doi.org/10.3390/nano10050840