Metal-Assembled Collagen Peptide Microflorettes as Magnetic Resonance Imaging Agents
Abstract
:1. Introduction
2. Results and Discussion
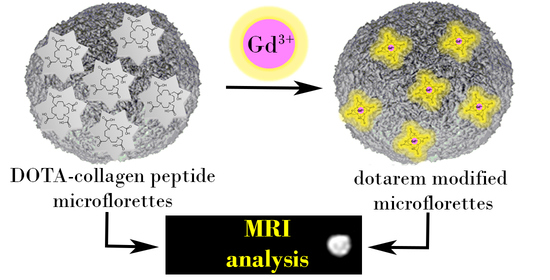
2.1. Design of MRI Active Collagen Peptide Microflorettes
2.2. Synthesis and Higher Order Assembly of Collagen Mimetic Peptides
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef]
- Meade, T.J.; Taylor, A.K.; Bull, S.R. New magnetic resonance contrast agents as biochemical reporters. Curr. Opin. Neurobiol. 2003, 13, 597–602. [Google Scholar] [CrossRef]
- Shuvaev, S.; Akam, E.; Caravan, P. Molecular MR Contrast Agents. Investig. Radiol. 2021, 56, 20–34. [Google Scholar] [CrossRef]
- Grover, V.P.; Tognarelli, J.M.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Doan, B.-T.; Meme, S.; Beloeil, J.-C. General Principles of MRI. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; Merbach, A., Helm, L., Tóth, É., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2013; pp. 1–23. [Google Scholar]
- Dumas, S.; Jacques, V.; Sun, W.C.; Troughton, J.S.; Welch, J.T.; Chasse, J.M.; Schmitt-Willich, H.; Caravan, P. High relaxivity magnetic resonance imaging contrast agents. Part 1. Impact of single donor atom substitution on relaxivity of serum albumin-bound gadolinium complexes. Investig. Radiol. 2010, 45, 600–612. [Google Scholar] [CrossRef] [Green Version]
- Botnar, R.M.; Brangsch, J.; Reimann, C.; Janssen, C.H.P.; Razavi, R.; Hamm, B.; Makowski, M.R. In Vivo Molecular Characterization of Abdominal Aortic Aneurysms Using Fibrin-Specific Magnetic Resonance Imaging. J. Am. Heart Assoc. 2018, 7, e007909. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Cai, Y.; Xu, Z.; Zhu, D. Preparation and Properties of Tumor-Targeting MRI Contrast Agent Based on Linear Polylysine Derivatives. Molecules 2019, 24, 1477. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Pan, D.; Schmieder, A.H.; Wickline, S.A.; Lanza, G.M. Manganese-based MRI contrast agents: Past, present, and future. Tetrahedron 2011, 67, 8431–8444. [Google Scholar] [CrossRef] [Green Version]
- Asik, D.; Smolinski, R.; Abozeid, S.M.; Mitchell, T.B.; Turowski, S.G.; Spernyak, J.A.; Morrow, J.R. Modulating the Properties of Fe(III) Macrocyclic MRI Contrast Agents by Appending Sulfonate or Hydroxyl Groups. Molecules 2020, 25, 2291. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Fasano, M.; Terreno, E. Lanthanide(III) chelates for NMR biomedical applications. Chem. Soc. Rev. 1998, 17, 19–29. [Google Scholar] [CrossRef]
- Werner, E.; Datta, A.; Jocher, C.; Raymond, K. High-Relaxivity MRI Contrast Agents: Where Coordination Chemistry Meets Medical Imaging. Angew. Chem. Int. Ed. 2008, 47, 8568–8580. [Google Scholar] [CrossRef] [Green Version]
- Jacques, V.; Dumas, S.; Sun, W.C.; Troughton, J.S.; Greenfield, M.T.; Caravan, P. High-relaxivity magnetic resonance imagng contrast agents. Part 2. Optimization of inner- and second-sphere relaxivity. Investig. Radiol. 2010, 45, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Clough, T.J.; Jiang, L.; Wong, K.-L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Kanal, E. Gadolinium based contrast agents (GBCA): Safety overview after 3 decades of clinical experience. Magn. Res. Imaging 2016, 34, 1341–1345. [Google Scholar] [CrossRef]
- Semelka, R.C.; Ramalho, J.; Vakharia, A.; AlObaidy, M.; Burke, L.M.; Jay, M.; Ramalho, M. Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn. Res. Imaging 2016, 34, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Yan, G.P.; Xu, W.; Yang, L.; Li, L.; Liu, F.; Guo, Q.-Z. Dextran Gadolinium Complexes as Contrast Agents for Magnetic Resonance Imaging to Sentinel Lymph Nodes. Pharm. Res. 2010, 27, 1884–1892. [Google Scholar] [CrossRef]
- Pierre, V.C.; Botta, M.; Raymond, K.N. Dendrimeric gadolinium chelate with fast water exchange and high relaxivity at high magnetic field strength. J. Am. Chem. Soc. 2005, 127, 504–505. [Google Scholar] [CrossRef]
- Huang, Y.; Coman, D.; Hyder, F.; Ali, M.M. Dendrimer-Based Responsive MRI Contrast Agents (G1–G4) for Biosensor Imaging of Redundant Deviation in Shifts (BIRDS). Bioconjug. Chem. 2015, 26, 2315–2323. [Google Scholar] [CrossRef] [Green Version]
- Caminade, A.-M.; Hameau, A.; Turrin, C.-O.; Laurent, R.; Majoral, J.-P. Dendritic metal complexes for bioimaging. Recent advances. Coord. Chem. Rev. 2021, 430, 213739. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, M.; Tong, X.; Sun, N.; Zhou, L.; Cao, Y.; Wang, J.; Zhang, H.; Pei, R. Aptamer-Modified Temperature-Sensitive Liposomal Contrast Agent for Magnetic Resonance Imaging. Biomacromolecules 2015, 16, 2618–2623. [Google Scholar] [CrossRef]
- Reeßing, F.; Stuart, M.C.A.; Samplonius, D.F.; Dierckx, R.A.J.O.; Feringa, B.L.; Helfrich, W.; Szymanski, W. A light-responsive liposomal agent for MRI contrast enhancement and monitoring of cargo delivery. Chem. Commun. 2019, 55, 10784–10787. [Google Scholar] [CrossRef]
- Kuijten, M.M.P.; Degeling, M.H.; Chen, J.W.; Wojtkiewicz, G.; Waterman, P.; Weissleder, R.; Azzi, J.; Nicolay, K.; Tannous, B.A. Multimodal targeted high relaxivity thermosensitive liposome for in vivo imaging. Sci. Rep. 2015, 5, 17220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manus, L.M.; Mastarone, D.J.; Waters, E.A.; Zhang, X.Q.; Schultz-Sikma, E.A.; MacRenaris, K.W.; Ho, D.; Meade, T.J. Gd(III)-Nanodiamond Conjugates for MRI Contrast Enhancement. Nano Lett. 2010, 10, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, S.M.; Robison, L.; Parigi, G.; Olszewski, A.; Drout, R.J.; Gong, X.; Islamoglu, T.; Luchinat, C.; Farha, O.K.; Meade, T.J. Maximizing Magnetic Resonance Contrast in Gd(III) Nanoconjugates: Investigation of Proton Relaxation in Zirconium Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 41157–41166. [Google Scholar] [CrossRef]
- Toth, E.; Bolskar, R.D.; Borel, A.; Gonzalez, G.; Helm, L.; Merbach, A.E.; Sitharaman, B.; Wilson, L.J. Water-soluble gadofullerenes: Toward high-relaxivity, pH-responsive MRI contrast agents. J. Am. Chem. Soc. 2005, 127, 799–805. [Google Scholar] [CrossRef]
- Babič, A.; Vorobiev, V.; Trefalt, G.; Crowe, L.A.; Helm, L.; Vallée, J.-P.; Allémann, E. MRI micelles self-assembled from synthetic gadolinium-based nano building blocks. Chem. Commun. 2019, 55, 945–948. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kokuryo, D.; Rafi, M.; Terada, Y.; Aoki, I.; Saga, T.; Takehiko, I.; Nishiyama, N.; Kataoka, K. Gd-DTPA-loaded polymeremetal complex micelles with high relaxivity for MR cancer imaging. Biomaterials 2013, 34, 492–500. [Google Scholar] [CrossRef]
- Caravan, P.; Greenwood, J.M.; Welch, J.T.; Franklin, S.J. Gadolinium-binding helix–turn–helix peptides: DNA-dependent MRI contrast agents. Chem. Commun. 2003, 20, 2574–2575. [Google Scholar] [CrossRef]
- Abiraj, K.; Jaccard, H.; Kretzschmar, M.; Helmb, L.; Maecke, H.R. Novel DOTA-based prochelator for divalent peptide vectorization: Synthesis of dimeric bombesin analogues for multimodality tumor imaging and therapy. Chem. Commun. 2008, 28, 3248–3250. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, V.; Gringeri, C.V.; Menchise, V.; Padovan, S.; Boffa, C.; Dastrù, W.; Chaabane, L.; Digilio, G.; Aime, S. A R2p/R1p Ratiometric Procedure to Assess Matrix Metalloproteinase-2 Activity by Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2013, 52, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yang, J.; Wei, L.; Zurkiya, O.; Yang, W.; Li, S.; Zou, J.; Zhou, Y.; Wilkins Maniccia, A.L.; Mao, H.; et al. Rational Design of Protein-Based MRI Contrast Agents. J. Am. Chem. Soc. 2008, 130, 9260–9267. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Wang, Y.; Zhang, T.; Pan, Y. Gadolinium-Labeled Ferritin Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging of Tumors. ACS Appl. Nano Mater. 2020, 3, 8771–8783. [Google Scholar] [CrossRef]
- Licciardi, G.; Rizzo, D.; Salobehaj, M.; Massai, L.; Geri, A.; Messori, L.; Ravera, E.; Fragai, M.; Parigi, G. Large Protein Assemblies for High-Relaxivity Contrast Agents: The Case of Gadolinium-Labeled Asparaginase. Bioconjug. Chem. 2022, 33, 2411–2419. [Google Scholar] [CrossRef]
- Villaraza, A.J.L.; Bumb, A.; Brechbiel, M.W. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef] [Green Version]
- Mulder, W.J.; Strijkers, G.J.; Griffioen, A.W.; van Bloois, L.; Molema, G.; Storm, G.; Koning, G.A.; Nicolay, K. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug. Chem. 2004, 15, 799–806. [Google Scholar] [CrossRef]
- Floyd III, W.C.; Klemm, P.J.; Smiles, D.E.; Kohlgruber, A.C.; Pierre, V.C.; Mynar, J.L.; Fréchet, J.M.J.; Raymond, K.N. Conjugation Effects of Various Linkers on Gd(III) MRI Contrast Agents with Dendrimers: Optimizing the Hydroxypyridinonate (HOPO) Ligands with Nontoxic, Degradable Esteramide (EA) Dendrimers for High Relaxivity. J. Am. Chem. Soc. 2011, 133, 2390–2393. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Corwin, F.D.; Zhang, J.; Chen, Z.; Reid, J.E.; Sun, M.; Xu, W.; Sim, J.H.; Wang, C.; Fatouros, P.P.; et al. Facile preparation of a new gadofullerene-based magnetic resonance imaging contrast agent with high 1H relaxivity. Bioconjug. Chem. 2009, 20, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Jastrzȩbska, B.; Lebel, R.; Therriault, H.; McIntyre, J.O.; Escher, E.; Guérin, B.; Paquette, B.; Neugebauer, W.A.; Lepage, M. New Enzyme-Activated Solubility-Switchable Contrast Agent for Magnetic Resonance Imaging: From Synthesis to in Vivo Imaging. J. Med. Chem. 2009, 52, 1576–1581. [Google Scholar] [CrossRef]
- Vymazal, J.; Spuentrup, E.; Cardenas-Molina, G.; Wiethoff, A.J.; Hartmann, M.G.; Caravan, P.; Parsons, E.C., Jr. Thrombus Imaging With Fibrin-Specific Gadolinium-Based MR Contrast Agent EP-2104R: Results of a Phase II Clinical Study of Feasibility. Investig. Radiol. 2009, 44, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.; Helm, P.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.-C.; et al. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angew. Chem. Int. Ed. 2007, 46, 8171–8173. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Kong, H.; He, P.; Yang, G.; Zhu, D.; Guo, L.; We, G. Self-Assembled Peptide-Based Nanodrugs: Molecular Design, Synthesis, Functionalization, and Targeted Tumor Bioimaging and Biotherapy. Small 2022, 19, 2205787. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Liang, G. Peptide-based supramolecular hydrogels for bioimaging applications. Biomater. Sci. 2021, 9, 315–327. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, X.; Liang, G.; Sun, X. Self-assembly of peptide nanofibers for imaging applications. Nanoscale 2021, 13, 15142–15150. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, Y.-L.; Ma, Z.-Y.; Han, K.; Han, H.-Y. Tumor-triggered transformation of chimeric peptide for dual-stage-amplified magnetic resonance imaging and precise photodynamic therapy. Biomaterials 2018, 182, 269–278. [Google Scholar] [CrossRef]
- Preslar, A.T.; Parigi, G.; McClendon, M.T.; Sefick, S.S.; Moyer, T.J.; Haney, C.R.; Waters, E.A.; MacRenaris, K.W.; Luchinat, C.; Stupp, S.I.; et al. Gd(III)-Labeled Peptide Nanofibers for Reporting on Biomaterial Localization in Vivo. ACS Nano 2014, 8, 7325–7332. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Han, E.H.; Ryu, J.; Min, J.-Y.; Ahn, H.; Chung, Y.-H.; Lee, E. One-Dimensional Supramolecular Nanoplatforms for Theranostics Based on Co-Assembly of Peptide Amphiphiles. Biomacromolecules 2016, 17, 3234–3243. [Google Scholar] [CrossRef]
- Vaccaro, M.; Mangiapia, G.; Paduano, L.; Gianolio, E.; Accardo, A.; Tesauro, D.; Morelli, G. Structural and Relaxometric Characterization of Peptide Aggregates Containing Gadolinium Complexes as Potential Selective Contrast Agents in MRI. ChemPhysChem 2007, 8, 2526–2538. [Google Scholar] [CrossRef]
- Chung, E.J.; Pineda, F.; Nord, K.; Karczmar, G.; Lee, S.-K.; Tirrell, M. Fibrin-Targeting, Peptide Amphiphile Micelles as Contrast Agents for Molecular MRI. J. Cell Sci. Ther. 2014, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.Y.; Shen, Y.Y.; Wang, J.D.; Liang, G.-L. Controlled intracellular self-assembly of gadolinium nanoparticles as smart molecular MR contrast agents. Sci. Rep. 2013, 3, 1024. [Google Scholar] [CrossRef] [Green Version]
- Sulek, S.; Mammadov, B.; Mahcicek, D.I.; Sozeri, H.; Atalar, E.; Tekinaya, A.B.; Guler, M.O. Peptide functionalized superparamagnetic iron oxide nanoparticles as MRI contrast agents. J. Mater. Chem. 2011, 21, 15157–15162. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, M.M.; Chmielewski, J. Self-assembly of collagen peptides into microflorettes via metal coordination. J. Am. Chem. Soc. 2009, 131, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Ernenwein, D.; Chmielewski, J. Selective Decoration and Release of His-tagged Proteins from Metal-Assembled Collagen Peptide Microflorettes. Biomacromolecules 2011, 12, 2429–2433. [Google Scholar] [CrossRef]
- Pires, M.M.; Lee, J.; Ernenwein, D.; Chmielewski, J. Controlling the morphology of metal-promoted higher ordered assemblies of collagen peptides with varied core lengths. Langmuir 2012, 28, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Persikov, A.V.; Ramshaw, J.A.; Kirkpatrick, A.; Brodsky, B. Amino Acid Propensities for the Collagen Triple-Helix. Biochemistry 2000, 39, 14960–14967. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.W.; Chmielewski, J. A comparison of the collagen triple helix and coiled-coil peptide building blocks on metal ion-mediated supramolecular assembly. Pept. Sci. 2021, 113, e224190. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Yu, J.; Luo, L.; Wang, J.; Xiao, J. Ln3+-Triggered self-assembly of a heterotrimer collagen mimetic peptide into luminescent nanofibers. Chem. Commun. 2020, 56, 15141–15144. [Google Scholar] [CrossRef]
- Yao, L.; Hu, Y.; Liu, Z.; Ding, X.; Tian, J.; Xiao, J. Luminescent Lanthanide–Collagen Peptide Framework for pH-Controlled Drug Delivery. Mol. Pharm. 2019, 16, 846–855. [Google Scholar] [CrossRef]
- Sun, X.; He, M.; Wang, L.; Luo, L.; Wang, J.; Xiao, J. Luminescent Biofunctional Collagen Mimetic Nanofibers. ACS Omega 2019, 4, 16270–16279. [Google Scholar] [CrossRef] [Green Version]
- Chan, O.C.M.; So, K.-F.; Chan, B.P. Fabrication of nano-fibrous collagen microspheres for protein delivery and effects of photochemical crosslinking on release kinetics. J. Control. Release 2008, 129, 135–143. [Google Scholar] [CrossRef]
- Mumcuoglu, D.; de Miguela, L.; Jekhmane, S.; Siverino, C.; Nickel, J.; Mueller, T.D.; van Leeuwen, J.P.; van Osch, G.J.; Kluijtmans, S.G. Collagen I derived recombinant protein microspheres as novel delivery vehicles for bone morphogenetic protein-2. Mater. Sci. Eng. C 2018, 84, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kachi-Terajima, C.; Yanagi, K.; Kaziki, T.; Kitazawa, T.; Hasegawa, M. Luminescence tuning of imidazole-based lanthanide(III) complexes [Ln = Sm, Eu, Gd, Tb, Dy]. Dalton Trans. 2011, 40, 2249–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachi-Terajima, C.; Shimoyama, T.; Ishigami, T.; Ikeda, M.; Habata, Y. A hemiaminal–ether structure stabilized by lanthanide complexes with an imidazole-based Schiff base ligand. Dalton Trans. 2018, 47, 2638–2645. [Google Scholar] [CrossRef] [PubMed]




| Peptide | [Zn] (µM) a | [Gd] (µM) a | Ratio [Zn]:[Gd] |
|---|---|---|---|
| NCoH | 0.165 | 0.003 | 55:1 |
| 5% NHdota | 0.173 | 0.111 | 1.6:1 |
| 5% DOTA-His6 | 0.156 | 0.158 | 1:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernenwein, D.; Geisler, I.; Pavlishchuk, A.; Chmielewski, J. Metal-Assembled Collagen Peptide Microflorettes as Magnetic Resonance Imaging Agents. Molecules 2023, 28, 2953. https://doi.org/10.3390/molecules28072953
Ernenwein D, Geisler I, Pavlishchuk A, Chmielewski J. Metal-Assembled Collagen Peptide Microflorettes as Magnetic Resonance Imaging Agents. Molecules. 2023; 28(7):2953. https://doi.org/10.3390/molecules28072953
Chicago/Turabian StyleErnenwein, Dawn, Iris Geisler, Anna Pavlishchuk, and Jean Chmielewski. 2023. "Metal-Assembled Collagen Peptide Microflorettes as Magnetic Resonance Imaging Agents" Molecules 28, no. 7: 2953. https://doi.org/10.3390/molecules28072953