Two New 4-Hydroxy-2-pyridone Alkaloids with Antimicrobial and Cytotoxic Activities from Arthrinium sp. GZWMJZ-606 Endophytic with Houttuynia cordata Thunb
Abstract
:1. Introduction
2. Results and Discussion
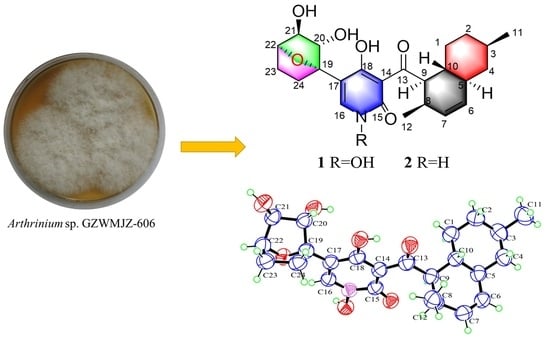
Structure Elucidation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation and Purification
3.5. Physical Properties and Spectral Data of 1–4
3.6. Antimicrobial Activities Assay
3.7. Cytotoxic Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tuson, R.V. XII.-Note on an Alkaloïd contained in the seeds of the Ricinus communis, or Castor-oil Plant. J. Chem. Soc. 1864, 17, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, S.; Minato, H.; Katagori, K. The ilicicolins antibiotics from Cylindrocladium ilicicola. J. Antibiot. 1971, 24, 653–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Zhang, X.; Feng, H.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Campyridones A-D, pyridone alkaloids from a mangrove endophytic fungus Campylocarpon sp. HDN13-307. Tetrahedron 2016, 72, 5679–5683. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhong, Y.; Yu, Y.; Shi, D.F.; Huang, H.Y.; Tang, X.L.; Wang, Y.H.; Chen, G.D.; Zhang, H.P.; Liu, C.L.; et al. 4-Hydroxy Pyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020, 83, 3338–3346. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Wang, L.; Cheng, Y.N.; Cao, Z.Q.; Zhang, X.K.; Guo, X.L. Discovery and Characterization of 4-Hydroxy-2-pyridone Derivative Sambutoxin as a Potent and Promising Anticancer Drug Candidate: Activity and Molecular Mechanism. Mol. Pharm. 2018, 15, 4898–4911. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Zhao, S. Progress in the Study of 4-Hydroxy-2-pyridone Natural Alkaloids. Chin. J. Org. Chem. 2011, 31, 9–21. [Google Scholar]
- Sarita, S.; Neelam, Y.; Ravi, K.; Sonu, C.; Vidhi, D.; Pooja, W.; Anil, D. A score years’ update in the synthesis and biological evaluation of medicinally important 2-pyridones. Eur. J. Med. Chem. 2022, 232, 114199. [Google Scholar]
- Ando, K.; Suzuki, S.; Saeki, T.; Tamura, G.; Arima, K. Funiculosin, A New Antibiotic. I Isolation, Biological and Chemical Properties. J. Antibiot. 1969, 22, 189–194. [Google Scholar] [CrossRef]
- Wat, C.K.; Mcinnes, A.G.; Smith, D.G.; Wright, J.L.C.; Vining, L.C. The yellow pigments of Beauveria species. Structures of tenellin and bassianin. Can. J. Chem. 1977, 55, 4090–4098. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Lee, Y.W.; Tamura, H.; Yoshizawa, T. Sambutoxin: A new mycotoxin isolated from Fusarium sambucinum. Tetrahedron Lett. 1995, 36, 1047–1050. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Supothina, S.; Tobwor, P.; Hywel-Jones, N.L. Pyridone and Tetramic Acid Alkaloids from the Spider Pathogenic FungusTorrubiella sp. BCC 2165. J. Nat. Prod. 2010, 73, 2057–2060. [Google Scholar] [CrossRef]
- Takahashi, S.; Kakinuma, N.; Uchida, K.; Hashimoto, R.; Yanagisawa, T.; Nakagawa, A. Pyridovericin and pyridomacrolidin: Novel metabolites from entomopathogenic fungi, Beauveria bassiana. J. Antibiot. 1998, 51, 596–598. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, A.; Qi, X.; Yu, R.; Li, J. A novel inhibitor of PGK1 suppresses the aerobic glycolysis and proliferation of hepatocellular carcinoma. Biomed. Pharmacother. 2023, 158, 114115. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Peng, C.; Ding, W.; Hu, J.F.; Li, J. Chromenopyridin A, a new N-methoxy-1-pyridone alkaloid from the endophytic fungus Penicillium nothofagi P-6 isolated from the critically endangered conifer Abies beshanzuensis. Nat. Prod. Res. 2022, 36, 2049–2055. [Google Scholar] [CrossRef]
- Chicca, A.; Berg, R.; Jessen, H.J.; Marck, N.; Schmind, F.; Burch, P.; Gertch, J.; Gademann, K. Biological evaluation of pyridone alkaloids on the endocannabinoid system. Bioorgan. Med. Chem. 2017, 25, 6102–6114. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Lu, H.; Zhang, Y.; Gan, X.; Wang, X.; Liu, Y.; Luo, X. Bioactive Alkaloids from the Beibu Gulf Coral-associated Fungus Acremonium sclerotigenum GXIMD 02501. Rec. Nat. Prod. 2023, 17, 165–169. [Google Scholar]
- Han, J.; Liu, C.; Li, L.; Zhou, H.; Liu, L.; Bao, L.; Chen, Q.; Song, F.; Zhang, L.; Li, E.; et al. Decalin-Containing Tetramic Acids and 4-Hydroxy-2-pyridones with Antimicrobial and Cytotoxic Activity from the Fungus Coniochaeta cephalothecoides Collected in Tibetan Plateau (Medog). J. Org. Chem. 2017, 82, 11474–11486. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Qin, X.; Lin, X.; Zhou, X.; Liao, S.; Yang, B.; Liu, J.; Tu, Z.; Liu, Y. Arthpyrones A−C, Pyridone Alkaloids from a Sponge-Derived Fungus Arthrinium arundinis ZSDS1-F3. Org. Lett. 2015, 17, 656–659. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Zhu, G.; Zuo, M.; Gong, Q.; He, W.; Li, M.; Yuan, C.; Hao, X.; Zhu, W. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019, 30, 431–434. [Google Scholar] [CrossRef]
- Williams, D.R.; Kammler, D.C.; Donnell, A.F.; Goundry, W.R.F. Total Synthesis of (+)-Apiosporamide: Assignment of Relative and Absolute Configuration. Angew. Chem. Int. Ed. 2005, 44, 6715–6718. [Google Scholar] [CrossRef]
- Bao, J.; Zhai, H.; Zhu, K.; Yu, J.H.; Zhang, Y.; Wang, Y.; Jiang, C.S.; Zhang, X.; Zhang, Y.; Zhang, H. Bioactive Pyridone Alkaloids from a Deep-Sea-Derived Fungus Arthrinium sp. UJNMF0008. Mar. Drugs 2018, 16, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Umeokoli, B.O.; Eze, P.; Heering, C.; Janiak, C.; Müller, W.E.G.; Orfali, R.S.; Hartmann, R.; Dai, H.; Lin, W.; et al. Secondary metabolites of the lichen-associated fungus Apiospora montagnei. Tetrahedron Lett. 2017, 58, 1702–1705. [Google Scholar] [CrossRef]
- Bockstahler, E.R.; Weaver, L.C.; Wright, D.L. 7-Oxabicyclo [2.2.1] heptane-2,3-dicarboximides with anticonvulsant activity. J. Med. Chem. 1968, 11, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.N.; Brown, B.R.; Sher, P.M.; Patel, M.M.; Hall, S.E.; Han, W.C.; Barrish, J.C.; Kocy, O.; Harris, D.N.; Goldenberg, H.J.; et al. Interphenylene 7-oxabicyclo [2.2.1] heptane oxazoles. Highly potent, selective, and long-acting thromboxane A2 receptor antagonists. J. Med. Chem. 1993, 36, 1401–1417. [Google Scholar] [CrossRef]
- Walter, W.G. Antitumor Imide Derivatives of 7-Oxabicyclo [2.2.1] heptane-2,3-dimethyl-2,3-dicarboxylic Acid. J. Pharm. Sci. 1989, 78, 66–67. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.; Wang, Z.; Fan, Y.; Yang, X.; Wang, Z.; Yu, S.; Pang, Q.; Cao, A. Deoxymikanolide adversely altered physiology and ultrastructure of Ralstonia solanacearum. Pestic. Biochem. Phys. 2021, 174, 104803. [Google Scholar] [CrossRef]
- Dockray, G.J. Cholecystokinins in rat cerebral cortex: Identification, purification and characterization by immunochemical methods. Brain Res. 1980, 188, 155–165. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [Green Version]
- Wufuer, H.; Xu, Y.; Wu, D.; He, W.; Wang, D.; Zhu, W.; Wang, L. Liglaurates A–E, cytotoxic bis (lauric acid-12yl) lignanoates from the rhizomes of Drynaria roosii Nakaike. Phytochemistry 2022, 198, 113143. [Google Scholar] [CrossRef]





| Position | 1 b | 2 a | 3 b | 4 b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 31.0, CH2 | 0.86–0.91, m 1.95, d (11.1) | 29.6, CH2 | 0.77–0.85, m 1.83, dd (12.2, 3.0) | 31.0, CH2 | 0.87–0.91, m 1.91–1.94, m | 31.0, CH2 | 0.86–0.92, m 1.90–1.95, m |
| 2 | 36.6, CH2 | 0.99–1.11, m 1.74, overlap | 35.1, CH2 | 0.92–1.00, m 1.67, d (12.1) | 36.6, CH2 | 1.00–1.09, m 1.73–1.77, overlap | 36.6, CH2 | 1.00–1.08, m 1.73–1.78, overlap |
| 3 | 34.4, CH | 1.48–1.53, m | 32.6, CH | 1.43–1.49, overlap | 34.3, CH | 1.49–1.52, m | 34.4, CH | 1.48–1.53, m |
| 4 | 43.2, CH2 | 0.80, t (12.2) 1.72–1.80, overlap | 41.4, CH2 | 0.72–0.78, overlap 1.69–1.72, overlap | 43.1, CH2 | 0.80, t (12.2) 1.73–1.77, overlap | 43.2, CH2 | 0.79, t (12.2) 1.73–1.78, overlap |
| 5 | 43.2, CH | 1.83, “t” like (10.8) | 41.4, CH | 1.75, “t” like (10.1) | 43.3, CH | 1.81–1.85, overlap | 43.2, CH | 1.80–1.86, overlap |
| 6 | 131.7, CH | 5.41, d (9.7) | 130.4, CH | 5.37, d (9.9) | 131.7, CH | 5.42, d (9.9) | 131.7, CH | 5.41, d (9.8) |
| 7 | 132.6, CH | 5.58–5.63, m | 131.7, CH | 5.56–5.59, m | 132.5, CH | 5.58–5.62, m | 132.6, CH | 5.60, ddd (9.8, 4.5, 2.7) |
| 8 | 32.3, CH | 2.83–2.90, m | 30.6, CH | 2.71–2.76, m | 32.3, CH | 2.82–2.86, m | 32.4, CH | 2.80–2.86, m |
| 9 | 54.5, CH | 4.42–4.47, overlap | 51.8, CH | 4.33, dd (11.4, 5.7) | 54.5, CH | 4.45, dd (11.3, 5.7) | 54.2, CH | 4.43, dd (11.4, 5.8) |
| 10 | 37.5, CH | 1.56–1.60, overlap | 35.8, CH | 1.41–1.49, m | 37.6, CH | 1.56–1.60, m | 37.6, CH | 1.54–1.60, m |
| 11 | 22.9, CH3 | 0.94, d (6.5) | 22.5, CH3 | 0.87, d (6.5) | 23.0, CH3 | 0.94, d (6.5) | 22.9, CH3 | 0.93, d (6.5) |
| 12 | 18.4, CH3 | 0.82, d (7.0) | 17.9, CH3 | 0.74, d (7.3) | 18.4, CH3 | 0.83, d (7.3) | 18.4, CH3 | 0.83, d (7.2) |
| 13 | 211.4, C | 209.6, C | 211.7, C | 212.0, C | ||||
| 14 | 108.2, C | 106.7, C | 108.6, C | 108.8, C | ||||
| 15 | 159.9, C | 161.8, C | 159.6, C | 163.9, C | ||||
| 16 | 139.5, CH | 7.93, s | 139.1, CH | 7.33, d (6.0) | 140.0, CH | 8.04, s | 139.9, CH | 7.58, s |
| 17 | 111.0, C | 110.8, C | 114.5, C | 116.6, C | ||||
| 18 | 173.2, C | 175.7, C | 175.6, C | 179.3, C | ||||
| 19 | 89.4, C | 88.0, C | 70.4, C | 70.4, C | ||||
| 20 | 82.1, CH | 3.87, brs | 80.5, CH | 3.58, dd (6.1, 1.4) | 60.5, CH | 3.66, d (3.7) | 60.5, CH | 3.64, “t” like (2.1) |
| 21 | 82.9, CH | 4.00, d (4.3) | 81.2, CH | 3.78, “t” like (4.7) | 57.7, CH | 3.43, “t” like (3.3) | 57.6, CH | 3.42, “t” like (3.3) |
| 22 | 78.6, CH | 4.42–4.47, overlap | 76.6, CH | 4.30, “t” like (5.1) | 67.2, CH | 4.12–4.14, m | 67.2, CH | 4.13, ddd (8.6, 5.7, 2.8) |
| 23 | 24.1, CH2 | 1.68–1.73, overlap 2.20–2.27, overlap | 23.0, CH2 | 1.45–1.54, m 2.02–2.10, overlap | 25.7, CH2 | 1.34–1.38, m 1.81–1.85, overlap | 25.8, CH2 | 1.32–1.38, m 1.80–1.86, overlap |
| 24 | 33.1, CH2 | 1.60–1.65, overlap 2.20–2.27, overlap | 31.8, CH2 | 1.45–1.49, overlap 2.01–2.08, overlap | 31.8, CH2 | 1.70, ddd (13.9, 10.2, 2.8) 2.26, ddd (14.4, 8.4, 2.5) | 31.6, CH2 | 1.70, ddd (13.5, 10.4, 2.5) 2.22, dd (13.3, 8.6) |
| 20-OH | 4.77, d (6.1) | |||||||
| 21-OH | 5.31, d (4.7) | |||||||
| -NH | 11.38, brs | |||||||
| Pathogenic Bacteria | 1 | 2 | 3 | 4 | Positive Drug |
|---|---|---|---|---|---|
| E. coli ATCC 11775 | >50 | >50 | >50 | >50 | 0.10 * |
| P. aeruginosa ATCC 10145 | >50 | >50 | >50 | >50 | 1.56 * |
| S. aureus ATCC6538 | 12.5 | >50 | 12.5 | 6.25 | 0.20 * |
| MRSA ATCC 43300 | 12.5 | >50 | 25.0 | 6.25 | 0.38 * |
| B. subtilis ATCC 6051 | >50 | >50 | >50 | 1.56 | 6.25 * |
| C. perfringens ATCC 13124 | >50 | >50 | >50 | 3.13 | 0.047 * |
| R. solanacarum | >50 | >50 | >50 | 6.25 | 3.12 * |
| C. albicans ATCC10231 | >50 | >50 | >50 | >50 | 3.13 # |
| C. glabrata ATCC2001 | >50 | >50 | >50 | >50 | 3.13 # |
| Cell Line | 1 | 2 | 3 | 4 | Dox |
|---|---|---|---|---|---|
| A549 | 6.47 ± 0.31 | >10 | >10 | >10 | 0.849 ± 0.013 |
| MKN-45 | 5.41 ± 0.09 | >10 | >10 | >10 | 0.307 ± 0.005 |
| HCT116 | 5.64 ± 0.05 | >10 | 6.09 ± 0.02 | >10 | 0.121 ± 0.005 |
| K562 | 9.22 ± 0.93 | >10 | >10 | >10 | 0.948 ± 0.058 |
| DU145 | 9.01 ± 0.07 | >10 | >10 | >10 | 0.189 ± 0.003 |
| SF126 | 9.72 ± 0.46 | >10 | >10 | >10 | 0.164 ± 0.016 |
| A-375 | 7.16 ± 0.17 | >10 | >10 | >10 | 0.064 ± 0.003 |
| 786-O | 5.93 ± 0.13 | >10 | 9.13 ± 0.48 | >10 | 0.726 ± 0.028 |
| PATU8988T | 6.46 ± 0.09 | >10 | >10 | >10 | 0.167 ± 0.012 |
| 5637 | 4.35 ± 0.08 | >10 | >10 | >10 | 0.185 ± 0.002 |
| HeLa | >10 | >10 | >10 | >10 | 0.177 ± 0.006 |
| TE-1 | >10 | >10 | >10 | >10 | 0.240 ± 0.030 |
| GBC-SD | >10 | >10 | >10 | >10 | 0.592 ± 0.069 |
| MCF-7 | >10 | >10 | >10 | >10 | 0.966 ± 0.011 |
| HepG2 | >10 | >10 | >10 | >10 | 0.619 ± 0.054 |
| CAL-62 | >10 | >10 | >10 | >10 | 0.277 ± 0.019 |
| HOS | >10 | >10 | >10 | >10 | 0.090 ± 0.013 |
| A-673 | >10 | >10 | >10 | >10 | 0.380 ± 0.030 |
| L-02 | 7.09 ± 0.10 | >10 | 9.70 ± 0.06 | >10 | 0.243 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wang, D.; Wu, D.; He, W.; Zuo, M.; Zhu, W.; Xu, Y.; Wang, L. Two New 4-Hydroxy-2-pyridone Alkaloids with Antimicrobial and Cytotoxic Activities from Arthrinium sp. GZWMJZ-606 Endophytic with Houttuynia cordata Thunb. Molecules 2023, 28, 2192. https://doi.org/10.3390/molecules28052192
Yin Y, Wang D, Wu D, He W, Zuo M, Zhu W, Xu Y, Wang L. Two New 4-Hydroxy-2-pyridone Alkaloids with Antimicrobial and Cytotoxic Activities from Arthrinium sp. GZWMJZ-606 Endophytic with Houttuynia cordata Thunb. Molecules. 2023; 28(5):2192. https://doi.org/10.3390/molecules28052192
Chicago/Turabian StyleYin, Ying, Dongyang Wang, Dan Wu, Wenwen He, Mingxing Zuo, Weiming Zhu, Yanchao Xu, and Liping Wang. 2023. "Two New 4-Hydroxy-2-pyridone Alkaloids with Antimicrobial and Cytotoxic Activities from Arthrinium sp. GZWMJZ-606 Endophytic with Houttuynia cordata Thunb" Molecules 28, no. 5: 2192. https://doi.org/10.3390/molecules28052192