Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Cell Assembly and Cathode Electrodes
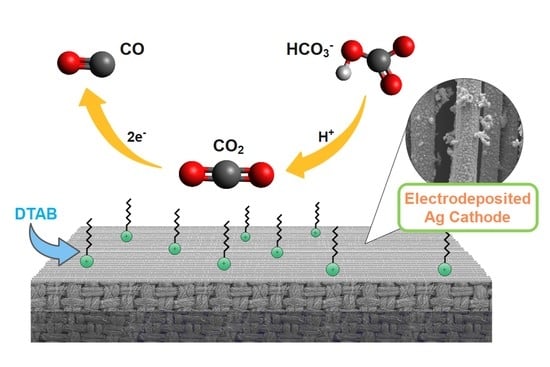
2.2. Cathode of Electrodeposited Ag
2.3. DTAB Surfactant in Catholyte
2.4. System Stability
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, J. Introduction to CO2 Electroreduction. In Electrochem. Reduct. Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Verma, S.; Kim, B.; Jhong, H.-R.; Ma, S.; Kenis, P.J.A. A Gross-Margin Model for Defining Technoeconomic Benchmarks in the Electroreduction of CO2. Chemsuschem 2016, 9, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Samavati, M.; Santarelli, M.; Martin, A.; Nemanova, V. Production of Synthetic Fischer–Tropsch Diesel from Renewables: Thermoeconomic and Environmental Analysis. Energy Fuels 2018, 32, 1744–1753. [Google Scholar] [CrossRef]
- Hernández, S.; Farkhondehfal, M.A.; Sastre, F.; Makkee, M.; Saracco, G.; Russo, N. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation. Green Chem. 2017, 19, 2326–2346. [Google Scholar] [CrossRef] [Green Version]
- Tountas, A.A.; Peng, X.; Tavasoli, A.V.; Duchesne, P.N.; Dingle, T.L.; Dong, Y.; Hurtado, L.; Mohan, A.; Sun, W.; Ulmer, U.; et al. Towards Solar Methanol: Past, Present, and Future. Adv. Sci. 2019, 6, 1801903. [Google Scholar] [CrossRef] [Green Version]
- Masel, R.I.; Liu, Z.; Yang, H.; Kaczur, J.J.; Carrillo, D.; Ren, S.; Salvatore, D.; Berlinguette, C.P. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 2021, 16, 118–128. [Google Scholar] [CrossRef]
- Salvatore, D.; Berlinguette, C.P. Voltage Matters When Reducing CO2 in an Electrochemical Flow Cell. ACS Energy Lett. 2019, 5, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Krause, R.; Reinisch, D.; Reller, C.; Eckert, H.; Hartmann, D.; Taroata, D.; Wiesner-Fleischer, K.; Bulan, A.; Lueken, A.; Schmid, G. Industrial Application Aspects of the Electrochemical Reduction of CO2 to CO in Aqueous Electrolyte. Chem. Ing. Tech. 2020, 92, 53–61. [Google Scholar] [CrossRef]
- Dinh, C.-T.; de Arquer, F.P.G.; Sinton, D.; Sargent, E.H. High Rate, Selective, and Stable Electroreduction of CO2 to CO in Basic and Neutral Media. ACS Energy Lett. 2018, 3, 2835–2840. [Google Scholar] [CrossRef]
- Haas, T.; Krause, R.; Weber, R.; Demler, M.; Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 2018, 1, 32–39. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Kutz, R.; Masel, R.I. CO2Electrolysis to CO and O2at High Selectivity, Stability and Efficiency Using Sustainion Membranes. J. Electrochem. Soc. 2018, 165, J3371–J3377. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Bhargava, S.S.; Proietto, F.; Azmoodeh, D.; Cofell, E.R.; Henckel, D.A.; Verma, S.; Brooks, C.J.; Gewirth, A.A.; Kenis, P.J.A. System Design Rules for Intensifying the Electrochemical Reduction of CO2 to CO on Ag Nanoparticles. Chemelectrochem 2020, 7, 2001–2011. [Google Scholar] [CrossRef]
- Jeng, E.; Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 2020, 5, 1768–1775. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The effect of electrolyte composition on the electroreduction of CO2to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2015, 18, 7075–7084. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2Reduction in a Flow Cell. Accounts Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Welch, A.J.; Dunn, E.; DuChene, J.S.; Atwater, H.A. Bicarbonate or Carbonate Processes for Coupling Carbon Dioxide Capture and Electrochemical Conversion. ACS Energy Lett. 2020, 5, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Endrődi, B.; Kecsenovity, E.; Samu, A.A.; Darvas, F.; Jones, R.V.; Török, V.; Danyi, A.; Janáky, C. Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency. ACS Energy Lett. 2019, 4, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Lee, G.; Yuan, T.; Wang, Y.; Nam, D.-H.; Wang, Z.; de Arquer, F.P.G.; Lum, Y.; Dinh, C.-T.; Voznyy, O.; et al. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4, 1427–1431. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic Conversion of Bicarbonate into CO in a Flow Cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Edwards, J.; Xu, Y.; Gabardo, C.M.; Dinh, C.-T.; Li, J.; Qi, Z.; Ozden, A.; Sargent, E.H.; Sinton, D. Efficient electrocatalytic conversion of carbon dioxide in a low-resistance pressurized alkaline electrolyzer. Appl. Energy 2019, 261, 114305. [Google Scholar] [CrossRef]
- Xie, K.; Miao, R.K.; Ozden, A.; Liu, S.; Chen, Z.; Dinh, C.-T.; Huang, J.E.; Xu, Q.; Gabardo, C.M.; Lee, G.; et al. Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products. Nat. Commun. 2022, 13, 3609. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.P.; Miao, R.K.; Liu, S.; Xu, Y.; Lee, G.; Robb, A.; Huang, J.E.; Xie, K.; Bertens, K.; Gabardo, C.M.; et al. Single Pass CO2 Conversion Exceeding 85% in the Electrosynthesis of Multicarbon Products via Local CO2 Regeneration. ACS Energy Lett. 2021, 6, 2952–2959. [Google Scholar] [CrossRef]
- Landaluce, N.; Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Luque, A.; Méndez, A.S.J.; Platero-Prats, A.E.; Pérez-Yáñez, S. Copper(II) invigorated EHU-30 for continuous electroreduction of CO2 into value-added chemicals. Sci. Rep. 2022, 12, 8505. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; Iniesta, J.; Irabien, A. Use of Chitosan as Copper Binder in the Continuous Electrochemical Reduction of CO2 to Ethylene in Alkaline Medium. Membranes 2022, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sainz, G.; Fernández-Caso, K.; Lagarteira, T.; Delgado, S.; Alvarez-Guerra, M.; Mendes, A.; Irabien, A. Coupling continuous CO2 electroreduction to formate with efficient Ni-based anodes. J. Environ. Chem. Eng. 2023, 11, 109171. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Castro, S.; Irabien, A.; Hernández, I.; Rodríguez, V.; Camarillo, R.; Rincón, J.; Albo, J. Efficient photoelectrochemical conversion of CO2 to ethylene and methanol using a Cu cathode and TiO2 nanoparticles synthesized in supercritical medium as photoanode. J. Environ. Chem. Eng. 2022, 10, 107441. [Google Scholar] [CrossRef]
- Azenha, C.; Mateos-Pedrero, C.; Alvarez-Guerra, M.; Irabien, A.; Mendes, A. Binary copper-bismuth catalysts for the electrochemical reduction of CO2: Study on surface properties and catalytic activity. Chem. Eng. J. 2022, 445, 136575. [Google Scholar] [CrossRef]
- Fernández-González, J.; Rumayor, M.; Domínguez-Ramos, A.I. CO2 electroreduction: Sustainability analysis of the renewable synthetic natural gas. Int. J. Greenh. Gas Control. 2022, 114, 103549. [Google Scholar] [CrossRef]
- Hoshi, N.; Kato, M.; Hori, Y. Electrochemical reduction of CO2 on single crystal electrodes of silver Ag(111), Ag(100) and Ag(110). J. Electroanal. Chem. 1997, 440, 283–286. [Google Scholar] [CrossRef]
- Benson, E.E.; Sampson, M.D.; Grice, K.A.; Smieja, J.M.; Froehlich, J.D.; Friebel, D.; Keith, J.A.; Carter, E.A.; Nilsson, A.; Kubiak, C.P. The Electronic States of Rhenium Bipyridyl Electrocatalysts for CO2Reduction as Revealed by X-ray Absorption Spectroscopy and Computational Quantum Chemistry. Angew. Chem. Int. Ed. 2013, 52, 4841–4844. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Cheng, C.; Chen, Z.; Schauer, C.K.; Meyer, T.J.; Brookhart, M. Selective Electrocatalytic Reduction of CO2 to Formate by Water-Stable Iridium Dihydride Pincer Complexes. J. Am. Chem. Soc. 2012, 134, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Tornow, C.E.; Thorson, M.R.; Ma, S.; Gewirth, A.A.; Kenis, P.J.A. Nitrogen-Based Catalysts for the Electrochemical Reduction of CO2 to CO. J. Am. Chem. Soc. 2012, 134, 19520–19523. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.; Wan, H.; Li, C.; An, W.; Sheng, X.; Liang, X.; Wang, X.; Ren, Y.; Zheng, X.; et al. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chin. Chem. Lett. 2021, 33, 2259–2269. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.; S, Kühl; Strasser, P.; Cuenya, B. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Rev. Mater. 2016, 7, 16009. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An overview of Cu-based heterogeneous electrocatalysts for CO2reduction. J. Mater. Chem. A 2020, 8, 4700–4734. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- de Arquer, F.G.; Dinh, C.-T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani, A.; Nam, D.-H.; Gabardo, C.; Seifitokaldani, A.; Wang, X.; et al. CO2 electrolysis to multicarbon products at activities greater than 1 A·cm−2. Science 2020, 367, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Hashimoto, K.; Hiramoto, M.; Watanabe, M.; Sakata, T. Electrochemical Reduction of Carbon Dioxide on Various Metal Electrodes in Low-Temperature Aqueous KHCO3 Media. J. Electrochem. Soc. 1990, 137, 1772–1778. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Song, S.; Liu, M.; Yao, S.; Liang, Z.; Cheng, H.; Ren, Z.; Liu, W.; Lin, R.; Qi, G.; et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage–A review. Petroleum 2018, 5, 335–340. [Google Scholar] [CrossRef]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Chueh, W.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.; Steinfeld, A. High-flux solar-driven thermochemical dis-sociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Accounts Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef]
- Sahara, G.; Ishitani, O. Efficient Photocatalysts for CO2 Reduction. Inorg. Chem. 2015, 54, 5096–5104. [Google Scholar] [CrossRef]
- Wang, Z.; Song, H.; Liu, H.; Ye, J. Coupling of Solar Energy and Thermal Energy for Carbon Dioxide Reduction: Status and Prospects. Angew. Chem. Int. Ed. 2020, 59, 8016–8035. [Google Scholar] [CrossRef]
- Wuttig, A.; Surendranath, Y. Impurity Ion Complexation Enhances Carbon Dioxide Reduction Catalysis. ACS Catal. 2015, 5, 4479–4484. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, D.A.; Weekes, D.M.; He, J.; Dettelbach, K.E.; Li, Y.C.; Mallouk, T.E.; Berlinguette, C.P. Electrolysis of Gaseous CO2 to CO in a Flow Cell with a Bipolar Membrane. ACS Energy Lett. 2017, 3, 149–154. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Smith, W.A. Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane. ACS Energy Lett. 2016, 1, 1143–1148. [Google Scholar] [CrossRef]
- Larrea, C.; Torres, D.; Avilés-Moreno, J.R.; Ocón, P. Multi-parameter study of CO2 electrochemical reduction from concentrated bicarbonate feed. J. CO2 Util. 2022, 57, 101878. [Google Scholar] [CrossRef]
- Zhang, Z.; Melo, L.; Jansonius, R.P.; Habibzadeh, F.; Grant, E.R.; Berlinguette, C.P. pH Matters When Reducing CO2 in an Electrochemical Flow Cell. ACS Energy Lett. 2020, 5, 3101–3107. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Habibzadeh, F.; Salvatore, D.A.; Ren, S.; Simpson, G.L.; Wheeler, D.G.; Liu, A.; Berlinguette, C.P. Porous metal electrodes enable efficient electrolysis of carbon capture solutions. Energy Environ. Sci. 2022, 15, 705–713. [Google Scholar] [CrossRef]
- Leonard, M.E.; Clarke, L.E.; Forner-Cuenca, A.; Brown, S.M.; Brushett, F.R. Investigating Electrode Flooding in a Flowing Electrolyte, Gas-Fed Carbon Dioxide Electrolyzer. Chemsuschem 2019, 13, 400–411. [Google Scholar] [CrossRef]
- Lees, E.W.; Mowbray, B.A.W.; Salvatore, D.A.; Simpson, G.L.; Dvorak, D.J.; Ren, S.; Chau, J.; Milton, K.L.; Berlinguette, C.P. Linking gas diffusion electrode composition to CO2 reduction in a flow cell. J. Mater. Chem. A 2020, 8, 19493–19501. [Google Scholar] [CrossRef]
- Quan, F.; Xiong, M.; Jia, F.; Zhang, L. Efficient electroreduction of CO2 on bulk silver electrode in aqueous solution via the inhibition of hydrogen evolution. Appl. Surf. Sci. 2017, 399, 48–54. [Google Scholar] [CrossRef]
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Kutz, R.B.; Chen, Q.; Yang, H.; Sajjad, S.D.; Liu, Z.; Masel, I.R. Sustainion Imidazolium-Functionalized Polymers for Carbon Dioxide Electrolysis. Energy Technol. 2017, 5, 929–936. [Google Scholar] [CrossRef] [Green Version]




| CO2 Feedstock | Catalyst | Membrane | J (mA·cm−2) | FECO | Temp | Pressure | Reference |
|---|---|---|---|---|---|---|---|
| 2M KHCO3 with 0.02M DTAB | Ag ED | BPM | 100 | 70% | 20 °C | 1 atm | This work |
| 200 | 45% | 20 °C | 1 atm | ||||
| 100 | 85% | 50 °C | 1 atm | ||||
| 200 | 73% | 50 °C | 1 atm | ||||
| 2M KHCO3 | Ag NP | BPM | 100 | 40% | 50 °C | 1 atm | [57] |
| 200 | 46% | 50 °C | 1 atm | ||||
| 3M KHCO3 | Ag XXXXXX(PVD + NP) | BPM | 100 | 82% | RT | 1 atm | [15] |
| 200 | 62% | RT | 1 atm | ||||
| 3M KHCO3 | Ag foam | BPM | 100 | 59% | 20 °C | 1 atm | [59] |
| 200 | 34% * | 20 °C | 1 atm | ||||
| 100 | 95% | 20 °C | 4 atm | ||||
| 100 | 78% | 70 °C | 1 atm | ||||
| 1M K2CO3 | Ag NP | BPM | 100 | 28% | [22] | ||
| 200 | 20% * | ||||||
| 3M KHCO3 | Ag NP | BPM | 100 | 37% | [23] | ||
| CO2(g) | Ag NP | BPM | 100 | 67% | [55] | ||
| 200 | 50% | ||||||
| CO2(g) | CoPc | AEM | 200 | 88% | [63] | ||
| CO2(g) | Ag NP | AEM | 200 | >90% | RT | [64] | |
| CO2(g) | Ag NP | - | 417 | 100% | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrea, C.; Avilés-Moreno, J.R.; Ocón, P. Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions. Molecules 2023, 28, 1951. https://doi.org/10.3390/molecules28041951
Larrea C, Avilés-Moreno JR, Ocón P. Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions. Molecules. 2023; 28(4):1951. https://doi.org/10.3390/molecules28041951
Chicago/Turabian StyleLarrea, Carlos, Juan Ramón Avilés-Moreno, and Pilar Ocón. 2023. "Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions" Molecules 28, no. 4: 1951. https://doi.org/10.3390/molecules28041951