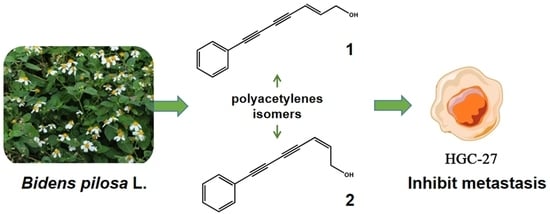
Polyacetylene Isomers Isolated from Bidens pilosa L. Suppress the Metastasis of Gastric Cancer Cells by Inhibiting Wnt/β-Catenin and Hippo/YAP Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Effects of Polyacetylenes 1 and 2 on Cell Viability
2.2. Determination of Non-Cytotoxic Concentrations
2.3. Effect of Treatment on the Wound-Healing Capacity of HGC-27 Cells
2.4. Effect of Treatment on the Migration and Invasion of HGC-27 Cells
2.5. Effect of Treatment on the Adhesion Ability of HGC-27 Cells
2.6. Polyacetylene 1 Treatment Reversed the EMT in HGC-27 Cells
2.7. Polyacetylene 1 Treatment Suppressed the Wnt/β-catenin Signaling Pathway in HGC-27 Cells
2.8. Polyacetylene 1 Treatment Suppressed the Hippo–YAP Signaling Pathway in HGC-27 Cells
3. Discussion
4. Materials and Methods
4.1. Cells and In Vitro Treatments
4.2. Chemical and Reagents
4.3. Cytotoxicity Evaluation
4.4. Clone Formation Assay
4.5. Wound-Healing Assay
4.6. Cell Adhesion Matrix Capability Assay
4.7. Transwell Migration/Invasion Assay
4.8. Immunofluorescence Assays
4.9. Western Blot Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Kakeji, Y.; Ishikawa, T.; Suzuki, S.; Akazawa, K.; Irino, T.; Miyashiro, I.; Ono, H.; Suzuki, H.; Tanabe, S.; Kadowaki, S.; et al. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001–2013). Gastric Cancer 2022, 25, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307–330. [Google Scholar] [CrossRef]
- Huels, D.J.; Ridgway, R.A.; Radulescu, S.; Leushacke, M.; Campbell, A.D.; Biswas, S.; Leedham, S.; Serra, S.; Chetty, R.; Moreaux, G.; et al. E-cadherin can limit the transforming properties of activating β-catenin mutations. EMBO J. 2015, 34, 2321–2333. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xi, H.; Cui, J.; Zhang, K.; Zhang, J.; Zhang, Y.; Xu, W.; Liang, W.; Zhuang, Z.; et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer 2020, 122, 1837–1847. [Google Scholar] [CrossRef]
- Zhou, H.; Li, G.; Huang, S.; Feng, Y.; Zhou, A. SOX9 promotes epithelial-mesenchymal transition via the Hippo-YAP signaling pathway in gastric carcinoma cells. Oncol. Lett. 2019, 18, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Zou, J.; Su, Y.; Wang, M.; Zhao, L. Resveratrol inhibits TGF-β1-induced EMT in gastric cancer cells through Hippo-YAP signaling pathway. Clin. Transl. Oncol. 2022, 24, 2210–2221. [Google Scholar] [CrossRef]
- Choi, W.; Kim, J.; Park, J.; Lee, D.H.; Hwang, D.; Kim, J.H.; Ashktorab, H.; Smoot, D.; Kim, S.Y.; Choi, C.; et al. YAP/TAZ Initiates Gastric Tumorigenesis via Upregulation of MYC. Cancer Res. 2018, 78, 3306–3320. [Google Scholar] [CrossRef]
- White, S.M.; Murakami, S.; Yi, C. The complex entanglement of Hippo-Yap/Taz signaling in tumor immunity. Oncogene 2019, 38, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Azzolin, L.; Panciera, T.; Soligo, S.; Enzo, E.; Bicciato, S.; Dupont, S.; Bresolin, S.; Frasson, C.; Basso, G.; Guzzardo, V.; et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 2014, 158, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Jho, E.H. Cross-talk between Wnt/β-catenin and Hippo signaling pathways: A brief review. BMB Rep. 2014, 47, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Konsavage, W.M., Jr.; Yochum, G.S. Intersection of Hippo/YAP and Wnt/β-catenin signaling pathways. Acta Biochim. Biophys. Sin. 2013, 45, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef]
- Kumari, P.; Misra, K.; Sisodia, B.S.; Faridi, U.; Srivastava, S.; Luqman, S.; Darokar, M.P.; Negi, A.S.; Gupta, M.M.; Singh, S.C.; et al. A promising anticancer and antimalarial component from the leaves of Bidens pilosa. Planta Med. 2009, 75, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Djebara, A.; Ciavatta, M.L.; Mathieu, V.; Colin, M.; Bitam, F.; Carbone, M.; Gavagnin, M. Oxygenated C(17) polyacetylene metabolites from Algerian Eryngium tricuspidatum L. roots: Structure and biological activity. Fitoterapia 2019, 138, 104355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Liu, R.; Jin, S.Q.; Fan, F.Y.; Zhan, Q.M. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006, 16, 356–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Senovilla, L.; Zitvogel, L.; Kroemer, G. The secret ally: Immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012, 11, 215–233. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.X.; Wang, Z.C.; Chen, X.Y.; Sun, Y.; Kong, Q.Y.; Liu, J.; Li, H. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: Relevance with tumour dissemination. Cancer Lett. 2005, 223, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Matsunaga, A.; Fujimura, T.; Tsukamoto, T.; Taketo, M.M.; Oshima, M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 2006, 131, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, T.; Murakami, H.; Fujii, M.; Ishiguro, F.; Tanaka, I.; Kondo, Y.; Akatsuka, S.; Toyokuni, S.; Yokoi, K.; Osada, H.; et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 2012, 31, 5117–5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Yimlamai, D.; Christodoulou, C.; Galli, G.G.; Yanger, K.; Pepe-Mooney, B.; Gurung, B.; Shrestha, K.; Cahan, P.; Stanger, B.Z.; Camargo, F.D. Hippo pathway activity influences liver cell fate. Cell 2014, 157, 1324–1338. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.G.; Bian, S.B.; Cui, J.X.; Xi, H.Q.; Zhang, K.C.; Qin, H.Z.; Zhu, X.M.; Chen, L. LKB1 inhibits the proliferation of gastric cancer cells by suppressing the nuclear translocation of Yap and β-catenin. Int. J. Mol. Med. 2016, 37, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.P.; Zhu, J.S.; Zhang, Q.; Wang, X.Y. A breakdown of the Hippo pathway in gastric cancer. Hepato-Gastroenterol. 2011, 58, 1611–1617. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.P.; Yang, Y.C.; Zhu, J.S.; Zhou, Z.; Chen, W.X. Expression of Yes-associated protein in gastric adenocarcinoma and inhibitory effects of its knockdown on gastric cancer cell proliferation and metastasis. Int. J. Immunopathol. Pharmacol. 2012, 25, 583–590. [Google Scholar] [CrossRef]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.L.; Menyiy, N.E.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A.; et al. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. Int. 2022, 29, 24411–24444. [Google Scholar] [CrossRef]
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 2014, 77, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Banyard, J.; Bielenberg, D.R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yook, J.I.; Li, X.Y.; Ota, I.; Fearon, E.R.; Weiss, S.J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005, 280, 11740–11748. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Feng, Y.; Hu, Y.; He, C.; Xie, C.; Ouyang, Y.; Artim, S.C.; Huang, D.; Zhu, Y.; Luo, Z.; et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. J. Exp. Clin. Cancer Res. 2018, 37, 280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Zhu, J.S.; Gao, C.P.; Li, L.P.; Zhou, C.; Wang, H.; Liu, X.G. siRNA targeting YAP gene inhibits gastric carcinoma growth and tumor metastasis in SCID mice. Oncol. Lett. 2016, 11, 2806–2814. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pan, P.; Wang, Z.; Zhang, Y.; Xie, P.; Geng, D.; Jiang, Y.; Yu, R.; Zhou, X. β-catenin-mediated YAP signaling promotes human glioma growth. J. Exp. Clin. Cancer Res. 2017, 36, 136. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, Y.C.; Yu, B.; Moroishi, T.; Mo, J.S.; Plouffe, S.W.; Meng, Z.; Lin, K.C.; Yu, F.X.; Alexander, C.M.; et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell 2015, 162, 780–794. [Google Scholar] [CrossRef]









| Cell Line | HGC-27 | MDA-MB-231 | HepG2 | BGC-823 | HCT-116 | Ca Ski |
|---|---|---|---|---|---|---|
| IC50 (μM) | 52.83 | 81.88 | 108.7 | 126.5 | 164.1 | 166.6 |
| Cell line | PANC-1 | A549 | NCI-N87 | AML12 | GES-1 | MDCK |
| IC50 (μM) | 171.4 | >200 | >200 | 73.92 | 160.6 | 171.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Shi, S.-Y.; Cheng, F.; Wei, M.; Zou, K.; Yu, X.-Q.; Chen, J.-F. Polyacetylene Isomers Isolated from Bidens pilosa L. Suppress the Metastasis of Gastric Cancer Cells by Inhibiting Wnt/β-Catenin and Hippo/YAP Signaling Pathways. Molecules 2023, 28, 1837. https://doi.org/10.3390/molecules28041837
Cai J, Shi S-Y, Cheng F, Wei M, Zou K, Yu X-Q, Chen J-F. Polyacetylene Isomers Isolated from Bidens pilosa L. Suppress the Metastasis of Gastric Cancer Cells by Inhibiting Wnt/β-Catenin and Hippo/YAP Signaling Pathways. Molecules. 2023; 28(4):1837. https://doi.org/10.3390/molecules28041837
Chicago/Turabian StyleCai, Jing, Song-Yun Shi, Fan Cheng, Min Wei, Kun Zou, Xiao-Qin Yu, and Jian-Feng Chen. 2023. "Polyacetylene Isomers Isolated from Bidens pilosa L. Suppress the Metastasis of Gastric Cancer Cells by Inhibiting Wnt/β-Catenin and Hippo/YAP Signaling Pathways" Molecules 28, no. 4: 1837. https://doi.org/10.3390/molecules28041837