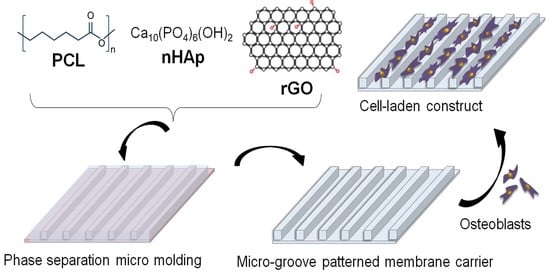
Evaluation of Human Osteoblasts on NIPS Micro-Patterned PCL Carriers Containing Nanohydroxyapatite and Reduced Graphene Oxide Using PSµM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Geometric Dimensions of Micromolds
2.3. Preparation and Optimization of Micropatterned Carriers
2.4. Morphological, Chemical and Structural Characterizations
2.4.1. Analysis of the Surface Morphology of Constructs via SEM
2.4.2. ATR-FTIR Analysis
2.4.3. XRD Analysis
2.5. Thermal and Physical Characterizations
2.5.1. DSC Analysis
2.5.2. TGA Analysis
2.5.3. Contact Angle Analysis
2.5.4. Porosity Analysis
- bulk volume (cm3) = thickness (cm) × length (cm) × width (cm)
- skeletal volume (cm3) = dry weight (g)/density (g/cm3)
2.5.5. Swelling Analysis
2.6. Mechanical Characterizations
2.6.1. Tensile Test
2.6.2. Compression Test
2.7. In Vitro Biological Characterizations
2.7.1. Indirect In Vitro Cytotoxicity Analysis
2.7.2. In Vitro Hemocompatibility Analysis
2.7.3. Seeding and Culturing of hOBs on the Micro-Patterned Constructs
MTT Assay
alamarBlue Assay
2.7.4. SEM Analysis of Cell-Laden Constructs
2.7.5. Calcium and ALP Analyses
2.7.6. von Kossa and Alizarin Red S Stainings
2.8. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology Analysis of Neat Constructs via SEM
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. Contact Angle Analysis
3.5. Porosity Analysis
3.6. Swelling Analysis
3.7. DSC Analysis
3.8. TGA Analysis
3.9. Compression Test
3.10. Tensile Test
3.11. In Vitro Hemocompatibility Test (ISO 10993-4)
3.12. Indirect In Vitro Cytotoxicity Test (ISO 10993-5)
3.13. SEM Analysis of Cell Laden Contructs
3.14. MTT Analysis
3.15. Time-Based alamarBlue Cell Viability Analysis
3.16. Osteogenic Activity of Cells on Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mat. Sci. Eng. C-Mater. 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-size bone defects: Is there a consensus for diagnosis and treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds-a review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, C.E.P.; Trujillo, S.; Demirdögen, B.; Jairo, E.; Perilla, J.E.; Elçin, Y.M.; Ribelles, J.L. New porous polycaprolactone-silica composites for bone regeneration. Mat. Sci. Eng. C-Mater. 2014, 40, 418–426. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Decellularized bovine small intestinal submucosa-PCL/hydroxyapatite-based multilayer composite scaffold for hard tissue repair. Mat. Sci. Eng. C-Mater. 2019, 94, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Osteogenic composite nanocoating based on nanohydroxyapatite, strontium ranelate and polycaprolactone for titanium implants. Trans. Nonferrous Met. Soc. China 2018, 28, 1763–1773. [Google Scholar] [CrossRef]
- Nalvuran, H.; Elçin, A.E.; Elçin, Y.M. Nanofibrous silk fibroin/reduced graphene oxide scaffolds for tissue engineering and cell culture applications. Int. J. Biol. Macromol. 2018, 114, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [PubMed]
- Pinar, E.; Sahin, A.; Unal, S.; Gunduz, O.; Harman, F.; Kaptanoglu, E. The effect of polycaprolactone/graphene oxide electrospun scaffolds on the neurogenic behavior of adipose stem cells. Eur. Polym. J. 2022, 165, 111000. [Google Scholar] [CrossRef]
- Carvalho, A.; Pelaez-Vargas, A.; Hansford, D.J.; Fernandes, M.H.; Monteiro, F.J. Effects of line and pillar array microengineered SiO2 thin films on the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Langmuir 2016, 32, 1091–1100. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol. Prog. 1998, 14, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kodama, Y.; Miwa, K.; Kishimoto, K.; Hoshikawa, E.; Haga, K.; Sato, T.; Mizuno, J.; Izumi, K. Manufacturing micropatterned collagen scaffolds with chemical-crosslinking for development of biomimetic tissue-engineered oral mucosa. Sci. Rep. 2020, 10, 22192. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Heinz, O.; Aghajani, M.; Greenberg, A.R.; Ding, Y. Surface-patterning of polymeric membranes: Fabrication and performance. Curr. Opin. Chem. Eng. 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef]
- Papenburg, B.J.; Liu, J.; Higuera, G.A.; Barradas, A.M.C.; de Boer, J.; van Blitterswijk, C.A.; Wessling, M.; Stamatialis, D. Development and analysis of multi-layer scaffolds for tissue engineering. Biomaterials 2009, 30, 6228–6239. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, V.; Pepe-Mooney, B.J. Understanding the cell behavior on nano-/micro-patterned surfaces. Nanomedicine 2012, 7, 1375–1389. [Google Scholar] [CrossRef] [Green Version]
- Gugutkov, D.; Awaja, F.; Belemezova, K.; Keramidarska, M.; Krasteva, N.; Kyurkchiev, S.; Gallego-Ferrer, G.; Seker, S.; Elçin, A.E.; Elçin, Y.M.; et al. Osteogenic differentiation of mesenchymal stem cells using hybrid nanofibers with different configurations and dimensionality. J. Biomed. Mater. Res. A 2017, 105, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Boehm, C.; Fleischman, A.J.; Muschler, G.F.; Roy, S. Connective tissue progenitor cell growth characteristics on textured substrates. Int. J. Nanomed. 2007, 2, 389–406. [Google Scholar] [PubMed]
- Lu, X.; Leng, Y. Comparison of the osteoblast and myoblast behavior on hydroxyapatite microgrooves. J. Biomed. Mater. Res. B 2009, 90, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xia, L.; Zhai, D.; Zhang, N.; Liu, J.; Fang, B.; Chang, J.; Lin, K. Designing ordered micropatterned hydroxyapatite bioceramics to promote the growth and osteogenic differentiation of bone marrow stromal cells. J. Mater. Chem. B 2015, 3, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Tüzün-Antepli, B.; Elçin, A.E.; Elçin, Y.M. Construction of micro-grooved PCL/nanohydroxyapatite membranes by non-solvent induced phase separation method and its evaluation for use as a substrate for human periodontal ligament fibroblasts. Chem. Eng. Sci. 2022, 248, 117120. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Part 5: Tests for in Vitro Cytotoxicity. Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009; pp. 1–34.
- Şeker, Ş.; Elçin, A.E.; Yumak, T.; Sinag, A.; Elçin, Y.M. In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. Vitr. 2014, 28, 1349–1358. [Google Scholar] [CrossRef]
- ISO 10993-4:2017; Part 4: Selection of Tests for Interactions with Blood. Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2017; pp. 1–36.
- Koc, A.; Elçin, A.E.; Elçin, Y.M. Ectopic osteogenic tissue formation by MC3T3-E1 cell-laden chitosan/hydroxyapatite composite scaffold. Artif. Cells Nanomed. B 2016, 44, 1440–1447. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci.-Mater. M 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Thinh, P.X.; Basavajara, C.; Kim, J.K.; Huh, D.S. Characterization and electrical properties of honeycomb-patterned poly (ε-caprolactone)/reduced graphene oxide composite film. Polym. Compos. 2012, 33, 2159–2168. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y. Study of reduced graphene oxide preparation by Hummers’ method and related characterization. J. Nanomater. 2015, 2015, 168125. [Google Scholar] [CrossRef]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Song, S.; Wang, J.; Nadimicherla, R.; Zhang, Z. Preparation and characterization of biodegradable poly (ε-caprolactone)-based gel polymer electrolyte films. Ionics 2015, 22, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Shahabi, S.; Najafi, F.; Majdabadi, A.; Hooshmand, T.; Nazarpak, M.H.; Karimi, B.; Fatemi, S.M. Effect of gamma ırradiation on structural and biological properties of a PLGA-PEG-hydroxyapatite composite. Sci. World J. 2014, 2014, 420616. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Nakhaei Moghaddam, M.R.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Hassan, M.I.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. 3 Biotech. 2017, 7, 249. [Google Scholar] [CrossRef] [Green Version]
- Ufere, S.K.J.; Sultana, N. Contact angle, conductivity and mechanical properties of polycaprolactone/hydroxyapatite/polypyrrole scaffolds using freeze-drying technique. ARPN J. Eng. Appl. Sci. 2016, 11, 13686–13691. [Google Scholar]
- Moffa, M.; Sciancalepore, A.G.; Passione, L.G.; Pisignano, D. Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and ımprinted micro-patterns. Small 2014, 10, 2439–2450. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Sarkar, K.; Chatterjee, K. Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 2016, 8, 6820–6836. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J. Mech. Behav. Biomed. 2017, 69, 115–127. [Google Scholar] [CrossRef]
- Basile, M.A.; d’Ayala, G.G.; Malinconico, M.; Laurienzo, P.; Coudane, J.; Nottelet, B.; Ragione, F.D.; Oliva, A. Functionalized PCL/HA nanocomposites as microporous membranes for bone regeneration. Mat. Sci. Eng. C-Mater. 2015, 48, 457–468. [Google Scholar] [CrossRef]
- Aerts, P.; Van Hoof, E.; Leysen, R.; Vankelecom, I.F.J.; Jacobs, P.A. Polysulfone–Aerosil composite membranes: Part 1. The influence of the addition of Aerosil on the formation process and membrane morphology. J. Membr. Sci. 2000, 176, 63–73. [Google Scholar] [CrossRef]
- Gohari, P.H.M.; Nazarpak, M.H.; Solati-Hashjin, M. The effect of adding reduced graphene oxide to electrospun polycaprolactone scaffolds on MG-63 cells activity. Mater. Today Commun. 2021, 27, 102287. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic degradation and mechanical stability of poly (ε-caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D Printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, K. Poly (ε-caprolactone)/hydroxyapatite composites: Effects of particle size, molecular weight distribution and irradiation on interfacial interaction and properties. Polym. Test. 2005, 24, 64–70. [Google Scholar] [CrossRef]
- Patlolla, A.; Collins, G.; Livingston Arinzeh, T. Solvent-dependent properties of electrospun fibrous composites for bone tissue regeneration. Acta Biomater. 2010, 6, 90–101. [Google Scholar] [CrossRef]
- Kim, M.J.; Koh, Y.H. Synthesis of aligned porous poly (ε-caprolactone) (PCL)/hydroxyapatite (HA) composite microspheres. Mat. Sci. Eng. C-Mater. 2013, 33, 2266–2272. [Google Scholar] [CrossRef]
- Diban, N.; Sánchez-González, S.; Lázaro-Díez, M.; Ramos-Vivas, J.; Urtiaga, A. Facile fabrication of poly (ε-caprolactone)/graphene oxide membranes for bioreactors in tissue engineering. J. Membr. Sci. 2017, 540, 219–228. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng.—T Asme 1999, 121, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lasgorceix, M.; Ott, C.; Boilet, L.; Hocquet, S.; Leriche, A.; Asadian, M.; De Geyter, N.; Declercq, H.; Lardot, V.; Cambier, F. Micropatterning of beta tricalcium phosphate bioceramic surfaces, by femtosecond laser, for bone marrow stem cells behavior assessment. Mat. Sci. Eng. C-Mater. 2019, 95, 371–380. [Google Scholar] [CrossRef] [PubMed]










| Pattern Code | C (Channel) | Ridge (R) | Depth (D) |
|---|---|---|---|
| 1 | 20 | 10 | 20 |
| 2 | 10 | 10 | 20 |
| Pattern Code | C (Channel) | Ridge (R) | Height (H) |
|---|---|---|---|
| 1 | 10 | 20 | 20 |
| 2 | 10 | 10 | 20 |
| Construct Code | PCL | HAp | rGO |
|---|---|---|---|
| P | 1.82 g | - | - |
| PH | 1.82 g | 0.364 g | - |
| PHG | 1.82 g | 0.364 g | 1 mg |
| Constructs | Pattern 1 | Pattern 2 | ||
|---|---|---|---|---|
| Channel | Ridge | Channel | Ridge | |
| P | 10.01 ± 0.45 | 17.24 ± 0.68 | 9.04 ± 0.81 | 8.83 ± 0.62 |
| PH | 9.47 ± 0.94 | 17.87 ± 0.86 | 8.33 ± 1.03 | 9.04 ± 0.64 |
| PHG | 9.12 ± 0.95 | 17.43 ± 0.81 | 8.80 ± 0.87 | 8.38 ± 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tüzün-Antepli, B.; Şeker, Ş.; Elçin, A.E.; Khang, G.; Elçin, Y.M. Evaluation of Human Osteoblasts on NIPS Micro-Patterned PCL Carriers Containing Nanohydroxyapatite and Reduced Graphene Oxide Using PSµM. Molecules 2022, 27, 7091. https://doi.org/10.3390/molecules27207091
Tüzün-Antepli B, Şeker Ş, Elçin AE, Khang G, Elçin YM. Evaluation of Human Osteoblasts on NIPS Micro-Patterned PCL Carriers Containing Nanohydroxyapatite and Reduced Graphene Oxide Using PSµM. Molecules. 2022; 27(20):7091. https://doi.org/10.3390/molecules27207091
Chicago/Turabian StyleTüzün-Antepli, Burcu, Şükran Şeker, Ayşe Eser Elçin, Gilson Khang, and Yaşar Murat Elçin. 2022. "Evaluation of Human Osteoblasts on NIPS Micro-Patterned PCL Carriers Containing Nanohydroxyapatite and Reduced Graphene Oxide Using PSµM" Molecules 27, no. 20: 7091. https://doi.org/10.3390/molecules27207091