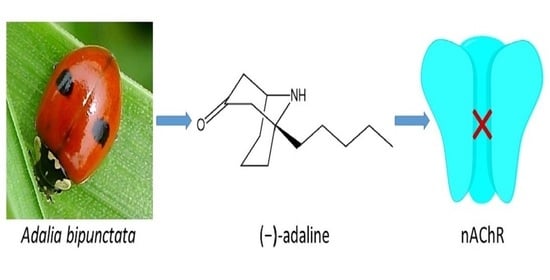
(−)-Adaline from the Adalia Genus of Ladybirds Is a Potent Antagonist of Insect and Specific Mammalian Nicotinic Acetylcholine Receptors
Abstract
:1. Introduction
2. Results
2.1. Quantification of Adalia Alkaloid Extracts and Their Effects on Human Muscle and Locust Neuronal nAChRs
2.2. (−)-Adaline Inhibits Natively Expressed nAChRs in a Non-Competitive and Voltage-Dependent Manner
2.3. Exploring Selectivity by Comparing Actions on Receptor Subtypes with Different Subunit Composition
2.4. Invertebrate Bioassays
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Nucleic Acids
4.2. Extraction of (−)-Adaline
4.3. TE671 Cell and Locust Neuron Culture
4.4. Whole-Cell Patch-Clamp Electrophysiology
4.5. Xenopus Laevis Oocyte Preparation and cRNA Injection
4.6. Two-Electrode, Voltage-Clamp Electrophysiology
4.7. Invertebrate Bioassays
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Happ, G.M.; Eisner, T. Hemorrhage in a Coccinellid Beetle and Its Repellent Effect on Ants. Science 1961, 134, 329. [Google Scholar] [CrossRef]
- Hemptinne, J.L.; Lognay, G.; Gauthier, C.; Dixon, A.F.G. Role of surface chemical signals in egg cannibalism and intraguild predation in ladybirds (Coleoptera: Coccinellidae). Chemoecology 2000, 10, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Phoofolo, M.W.; Obrycki, J.J. Potential for intraguild predation and competition among predatory Coccinellidae and Chrysopidae. Entomol. Exp. Appl. 1998, 89, 47–55. [Google Scholar] [CrossRef]
- Santi, F.; Maini, S. Predation upon adalia bipunctata and harmonia axyridis eggs by chrysoperla carnea larvae and orius laevigatus adults. Bull. Insectol. 2006, 59, 53–58. [Google Scholar]
- Marples, N.M.; Brakefield, P.M.; Cowie, R.J. Differences between the 7-Spot and 2-Spot Ladybird Beetles (Coccinellidae) in Their Toxic Effects on a Bird Predator. Ecol. Entomol. 1989, 14, 79–84. [Google Scholar] [CrossRef]
- Haulotte, E.; Laurent, P.; Braekman, J.C. Biosynthesis of Defensive Coccinellidae Alkaloids: Incorporation of Fatty Acids in Adaline, Coccinelline, and Harmonine. Eur. J. Org. Chem. 2012, 2012, 1907–1912. [Google Scholar] [CrossRef]
- Laurent, P.; Braekman, J.C.; Daloze, D.; Pasteels, J.M. In Vitro production of adaline and coccinelline, two defensive alkaloids from ladybird beetles (Coleoptera: Coccinellidae). Insect Biochem. Mol. Biol. 2002, 32, 1017–1023. [Google Scholar] [CrossRef]
- Majerus, M.E.N. A Natural History of Ladybird Beetles; Cambridge University Press: Cambridge, UK, 2016; pp. 1–397. [Google Scholar] [CrossRef]
- Umana, I.C.; Daniele, C.A.; McGehee, D.S. Neuronal nicotinic receptors as analgesic targets: It’s a winding road. Biochem. Pharmacol. 2013, 86, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Interference of alkaloids with neuroreceptors and ion channels. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, pp. 3–122. [Google Scholar]
- Leong, R.L.; Xing, H.; Braekman, J.C.; Kem, W.R. Non-competitive Inhibition of Nicotinic Acetylcholine Receptors by Ladybird Beetle Alkaloids. Neurochem. Res. 2015, 40, 2078–2086. [Google Scholar] [CrossRef]
- Alujas-Burgos, S.; Bayon, P.; Figueredo, M. Recent advances in the synthesis of azaphenalene alkaloids: First enantioselective approaches. Org. Biomol. Chem. 2018, 16, 8218–8229. [Google Scholar] [CrossRef] [PubMed]
- Alujas-Burgos, S.; Oliveras-Gonzalez, C.; Alvarez-Larena, A.; Bayon, P.; Figueredo, M. Iterative Synthetic Strategy for Azaphenalene Alkaloids. Total Synthesis of (-)-9a epi-Hippocasine. J. Org. Chem. 2018, 83, 5052–5057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.N.; Richards, D.P.; Duce, I.R.; Birkett, M.A.; Sattelle, D.B.; Mellor, I.R. Actions on mammalian and insect nicotinic acetylcholine receptors of harmonine-containing alkaloid extracts from the harlequin ladybird Harmonia axyridis. Pestic. Biochem. Phys. 2020, 166, 104561. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C.R. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Changeux, J.-P.; Edelstein, S.J. Allosteric receptors after 30 years. Rend. Lincei 2006, 17, 59. [Google Scholar] [CrossRef]
- Wooltorton, J.R.A.; Pidoplichko, V.I.; Broide, R.S.; Dani, J.A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 2003, 23, 3176–3185. [Google Scholar] [CrossRef]
- Kalamida, D.; Poulas, K.; Avramopoulou, V.; Fostieri, E.; Lagoumintzis, G.; Lazaridis, K.; Sideri, A.; Zouridakis, M.; Tzartos, S.J. Muscle and neuronal nicotinic acetylcholine receptors—Structure, function and pathogenicity. FEBS J. 2007, 274, 3799–3845. [Google Scholar] [CrossRef]
- Breer, H.; Sattelle, D.B. Molecular-Properties and Functions of Insect Acetylcholine-Receptors. J. Insect Physiol. 1987, 33, 771–790. [Google Scholar] [CrossRef]
- Gepner, J.I.; Hall, L.M.; Sattelle, D.B. Insect Acetylcholine Receptors as a Site of Insecticide Action. Nature 1978, 276, 188–190. [Google Scholar] [CrossRef]
- Ihara, M.; Hikida, M.; Matsushita, H.; Yamanaka, K.; Kishimoto, Y.; Kubo, K.; Watanabe, S.; Sakamoto, M.; Matsui, K.; Yamaguchi, A.; et al. Loops D, E and G in the Drosophila D1 subunit contribute to high neonicotinoid sensitivity of D1-chicken 2 nicotinic acetylcholine receptor. Br. J. Pharmacol. 2018, 175, 1999–2012. [Google Scholar] [CrossRef] [Green Version]
- Sattelle, D.B. Acetylcholine Receptors of Insects. In Advances in Insect Physiology; Berridge, M.J., Treherne, J.E., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1980; Volume 15, pp. 215–315. [Google Scholar]
- Lee, S.J.; Tomizawa, M.; Casida, J.E. Nereistoxin and cartap neurotoxicity attributable to direct block of the insect nicotinic receptor/channel. J. Agric. Food Chem. 2003, 51, 2646–2652. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Harrow, I.D.; David, J.A.; Pelhate, M.; Callec, J.J.; Gepner, J.I.; Hall, L.M. Nereistoxin—Actions on a Cns Acetylcholine-Receptor Ion Channel in the Cockroach Periplaneta-Americana. J. Exp. Biol. 1985, 118, 37–52. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crouse, G.D.; Durst, G. Natural products as insecticides: The biology, biochemistry, and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag. Sci. 2001, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kanaoka, S.; Akamatsu, M.; Sattelle, D.B. Diverse Actions and Target-Site Selectivity of Neonicotinoids: Structural Insights. Mol. Pharmacol. 2009, 76, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wolfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, E.E.; Schleicher, S.; Buschges, A.; Schmidt, J.; Kloppenburg, P.; Salgado, V.L. Desensitization of nicotinic acetylcholine receptors in central nervous system neurons of the stick insect (Carausius morosus) by imidacloprid and sulfoximine insecticides. Insect Biochem. Mol. Biol. 2011, 41, 872–880. [Google Scholar] [CrossRef]
- Watson, G.B.; Loso, M.R.; Babcock, J.M.; Hasler, J.M.; Letherer, T.J.; Young, C.D.; Zhu, Y.M.; Casida, J.E.; Sparks, T.C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 2011, 41, 432–439. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Schroeder, M.E.; Holyoke, C.W.; Zhang, W.; Pahutski, T.F.; Leighty, R.M.; Vincent, D.R.; Hamm, J.C. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 2016, 74, 32–41. [Google Scholar] [CrossRef]
- Thompson, G.D.; Dutton, R.; Sparks, T.C. Spinosad—A case study: An example from a natural products discovery programme. Pest Manag. Sci. 2000, 56, 696–702. [Google Scholar] [CrossRef]
- Brier, T.J.; Mellor, I.R.; Tikhonov, D.B.; Neagoe, I.; Shao, Z.Y.; Brierley, M.J.; Stromgaard, K.; Jaroszewski, J.W.; Krogsgaard-Larsen, P.; Usherwood, P.N.R. Contrasting actions of philanthotoxin-343 and philanthotoxin(12) on human muscle nicotinic acetylcholine receptors. Mol. Pharmacol. 2003, 64, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Woodhull, A.M. Ionic Blockage of Sodium Channels in Nerve. J. Gen. Physiol. 1973, 61, 687–708. [Google Scholar] [CrossRef]
- Choudhary, S.; Kashyap, S.S.; Martin, R.J.; Robertson, A.P. Advances in our understanding of nematode ion channels as potential anthelmintic targets. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 52–86. [Google Scholar] [CrossRef] [PubMed]
- Crossthwaite, A.J.; Bigot, A.; Camblin, P.; Goodchild, J.; Lind, R.J.; Slater, R.; Maienfisch, P. The invertebrate pharmacology of insecticides acting at nicotinic acetylcholine receptors. J. Pestic. Sci. 2017, 42, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luck, V.L.; Richards, D.P.; Shaikh, A.Y.; Franzyk, H.; Mellor, I.R. The Effects of Structural Alterations in the Polyamine and Amino Acid Moieties of Philanthotoxins on Nicotinic Acetylcholine Receptor Inhibition in the Locust, Schistocerca gregaria. Molecules 2021, 26, 7007. [Google Scholar] [CrossRef] [PubMed]
- Mellor, I.R.; Brier, T.J.; Pluteanu, F.; Stromgaard, K.; Saghyan, A.; Eldursi, N.; Brierley, M.J.; Anderson, K.; Jaroszewski, J.W.; Krogsgaard-Larsen, P.; et al. Modification of the philanthotoxin-343 polyamine moiety results in different structure-activity profiles at muscle nicotinic ACh, NMDA and AMPA receptors. Neuropharmacology 2003, 44, 70–80. [Google Scholar] [CrossRef]
- Rozental, R.; Scoble, G.T.; Albuquerque, E.X.; Idriss, M.; Sherby, S.; Sattelle, D.B.; Nakanishi, K.; Konno, K.; Eldefrawi, A.T.; Eldefrawi, M.E. Allosteric Inhibition of Nicotinic Acetylcholine-Receptors of Vertebrates and Insects by Philanthotoxin. J. Pharmacol. Exp. Ther. 1989, 249, 123–130. [Google Scholar]
- Kachel, H.S.; Patel, R.N.; Franzyk, H.; Mellor, I.R. Block of nicotinic acetylcholine receptors by philanthotoxins is strongly dependent on their subunit composition. Sci. Rep. 2016, 6, 38116. [Google Scholar] [CrossRef]
- Iperti, G. Biodiversity of predaceous coccinellidae in relation to bioindication and economic importance. Agric. Ecosyst. Environ. 1999, 74, 323–342. [Google Scholar] [CrossRef]
- Durieux, D.; Verheggen, F.; Vandereycken, A.; Joie, E.; Haubruge, É. Synthèse bibliographique: L’écologie chimique des coccinelles. Biotechnol. Agron. Société Environ. 2010, 14, 351–367. [Google Scholar]
- King, A.G.; Meinwald, J. Review of the defensive chemistry of coccinellids. Chem. Rev. 1996, 96, 1105–1122. [Google Scholar] [CrossRef]
- Lognay, G.; Hemptinne, J.L.; Chan, F.Y.; Gaspar, C.H.; Marlier, M.; Braekman, J.C.; Daloze, D.; Pasteels, J.M. Adalinine, a new piperidine alkaloid from the ladybird beetles Adalia bipunctata and Adalia decempunctata. J. Nat. Prod. 1996, 59, 510–511. [Google Scholar] [CrossRef]
- Raal, A.; Meos, A.; Hinrikus, T.; Heinamaki, J.; Romane, E.; Gudiene, V.; Jak Tas, V.; Koshovyi, O.; Kovaleva, A.; Fursenco, C.; et al. Dragendorff’s reagent: Historical perspectives and current status of a versatile reagent introduced over 150 years ago at the University of Dorpat, Tartu, Estonia. Pharmazie 2020, 75, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.A.; Schoepfer, R.; Whiting, P.; Casey, B.; Blatt, Y.; Montal, M.S.; Montal, M.; Lindstrom, J. A Muscle Acetylcholine-Receptor Is Expressed in the Human Cerebellar Medulloblastoma Cell-Line Te671. J. Neurosci. 1989, 9, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Dascal, N.; Landau, E.M. Types of Muscarinic Response in Xenopus Oocytes. Life Sci. 1980, 27, 1423–1428. [Google Scholar] [CrossRef]
- Khambay, B.P.; Batty, D.; Cahill, M.; Denholm, I.; Mead-Briggs, M.; Vinall, S.; Niemeyer, H.M.; Simmonds, M.S. Isolation, characterization, and biological activity of naphthoquinones from Calceolaria andina L. J. Agric. Food Chem. 1999, 47, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Glick, S.D.; Maisonneuve, I.M.; Kitchen, B.A. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur. J. Pharmacol. 2002, 448, 185–191. [Google Scholar] [CrossRef]
- Toll, L.; Zaveri, N.T.; Polgar, W.E.; Jiang, F.; Khroyan, T.V.; Zhou, W.; Xie, X.S.; Stauber, G.B.; Costello, M.R.; Leslie, F.M. AT-1001: A high affinity and selective alpha3beta4 nicotinic acetylcholine receptor antagonist blocks nicotine self-administration in rats. Neuropsychopharmacology 2012, 37, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Glick, S.D.; Maisonneuve, I.M.; Kitchen, B.A.; Fleck, M.W. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur. J. Pharmacol. 2002, 438, 99–105. [Google Scholar] [CrossRef]







| Cell/nAChR Type | IC50, µg/mL (95% CI) | Peak/1-s Ratio | |
|---|---|---|---|
| Peak Current | 1-s Current | ||
| TE671 (human muscle-type) | 48.4 (13.9–169) | 10.2 (7.18–14.5) | 4.75 |
| Locust (insect neuronal-type) | 3.66 (2.38–5.63) | 0.29 (0.24–0.33) | 12.6 |
| Cell/nAChR Type | VH (mV) | IC50, µM (95% CI) | Selectivity | |
|---|---|---|---|---|
| Peak Current | 1-s Current | |||
| TE671 (human muscle-type) | +50 | >>100 | >>100 | - |
| −50 | >100 | 49.2 (38.1–68.0) | ||
| −75 | >100 | 25.4 (16.2–41.1) | ||
| −100 | >100 | 13.2 (8.17–20.3) | ||
| −120 | >100 | 11.5 (6.38–19.2) | ||
| Locust (insect neuronal-type) | −50 | >100 | 2.97 (1.92–4.51) | 17 |
| −75 | >100 | 1.28 (0.83–1.97) | 20 | |
| −100 | 50.3 (33.3–94.8) | 0.70 (0.44–1.10) | 19 | |
| −120 | 53.4 (32.9–146) | 0.55 (0.32–0.91) | 21 | |
| Current | EC50, µM (95% CI) | p-Value | |
|---|---|---|---|
| ACh Alone | +20 μM (−)-Adaline | ||
| Peak | 1.43 (1.26–1.64) | 1.70 (1.37–2.26) | 0.171 |
| 1-s | 1.27 (1.14–1.42) | 1.43 (1.15–1.83) | 0.308 |
| Receptor | IC50, μM (95% CI) | |
|---|---|---|
| Peak Current | 15-s Current | |
| α4β2 | 24.8 (16.2–46.2) | 14.0 (9.76–22.0) |
| α3β4 | 0.22 (0.10–0.47) | 0.10 (0.047–0.21) |
| α7 | 10.4 (4.61–29.9) | N/A |
| Dα2/Gβ2 | 2.84 (1.60–5.03) | N/A |
| Species | Activity |
|---|---|
| House fly | 0% a |
| Mustard beetle | 85% a |
| Tobacco whitefly (SUD-S) | 12 ppm b |
| Tobacco whitefly (ISR-R) | 26 ppm b |
| Green peach aphid | 1% c |
| Red spider mite | 0% c |
| Diamondback moth | 3% d |
| Diamondback moth | 90% e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, D.P.; Patel, R.N.; Duce, I.R.; Khambay, B.P.S.; Birkett, M.A.; Pickett, J.A.; Mellor, I.R. (−)-Adaline from the Adalia Genus of Ladybirds Is a Potent Antagonist of Insect and Specific Mammalian Nicotinic Acetylcholine Receptors. Molecules 2022, 27, 7074. https://doi.org/10.3390/molecules27207074
Richards DP, Patel RN, Duce IR, Khambay BPS, Birkett MA, Pickett JA, Mellor IR. (−)-Adaline from the Adalia Genus of Ladybirds Is a Potent Antagonist of Insect and Specific Mammalian Nicotinic Acetylcholine Receptors. Molecules. 2022; 27(20):7074. https://doi.org/10.3390/molecules27207074
Chicago/Turabian StyleRichards, David P., Rohit N. Patel, Ian R. Duce, Bhupinder P. S. Khambay, Michael A. Birkett, John A. Pickett, and Ian R. Mellor. 2022. "(−)-Adaline from the Adalia Genus of Ladybirds Is a Potent Antagonist of Insect and Specific Mammalian Nicotinic Acetylcholine Receptors" Molecules 27, no. 20: 7074. https://doi.org/10.3390/molecules27207074