The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments
Abstract
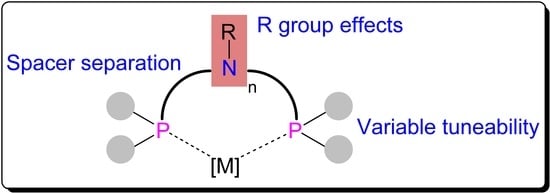
:1. Introduction
2. P–N Chemistry
2.1. Synthesis of Selected Examples
2.2. Importance of –N(H)– Backbone Functionality in P–N Ligands
3. P–N–P Chemistry
3.1. Synthesis of Selected Examples
3.2. Importance of –N(R)– Backbone Functionality in P–N–P Ligands
4. Bicyclic P–N Chemistry
5. P–C–N Chemistry
5.1. Synthesis of Selected Examples
5.2. Importance of –N(H)– Backbone Functionality in P–C–N Ligands
6. PTA Chemistry
6.1. Synthesis of Selected Examples
6.2. Importance of -N- Backbone Functionality in PTA and Related Compounds
7. P–C–N–C–P Chemistry
7.1. Synthesis of Selected Examples
7.2. Importance of –N(R)– Backbone Functionality in P–C–N–C–P Ligands
8. P–C–N–P and P–N–N–P Ligands
Synthesis of Selected Examples
9. P–C–C–N–C–C–P Ligands
9.1. Synthesis of Selected Examples
9.2. Importance of –N(R)– Backbone Functionality in P–C–C–N–C–C–P Ligands
10. Small/Medium Ring Based Cyclic Ligands
10.1. Synthesis of Selected Examples
10.2. Importance of –N(R)– Backbone Functionality in Cyclic Diphosphines
11. P–C–P–C–N–C–P–C–P and P–C2–N–C2–N–C2–P Ligands
11.1. Synthesis of Selected Examples
11.2. Importance of –N(R)– Backbone Functionality
| Ligand | δ(P)/ppm | NMR Solvent | Reference |
|---|---|---|---|
| 1a | 25.9 | CDCl3 | [25] |
| 1b | 26.4 | CDCl3 | [11] |
| 1c | 38.5 | CDCl3 | [12] |
| 1d | 42.2 | CDCl3 | [13] |
| 1e | 42.1 | CDCl3 | [13] |
| 1f | 26.1 | CDCl3 | [13] |
| 1g | 71.3 | CDCl3 | [14] |
| 1j | 32.6 (and 12.6) | C6D6 | [17] |
| 1k | 63.9 | C6D6 | [18] |
| 1l | 106.4 | CDCl3 | [19] |
| 1m | 77.9 | C6D6 | [20] |
| 1n | 126 | CD3CN | [23] |
| dppa | 43.1 | CDCl3 | [31] |
| 2a | 50.1 | CDCl3 | [32] |
| 2b | 62.1 | CDCl3 | [35] |
| 2f | −6.4 | CD2Cl2 | [39] |
| 2g | 69.0 | CDCl3 | [41] |
| 2ha | 137.9/135.3 | C7D8 | [42] |
| 2i | 69.3 | CDCl3 | [44] |
| 2m | 59.5 | CDCl3 | [52] |
| 2n | 17.2 and −20.0 (JPP 277 Hz) | CD2Cl2 | [52] |
| 3a | 159.8 | CDCl3 | [55] |
| 3c | −77.0 | CDCl3 | [56] |
| 4a | −16.7 | C6D6 | [57] |
| 4b | −19.4 | C6D6 | [57] |
| 4c | −17.1 | CDCl3 | [58] |
| 4d | −18.6 | CDCl3 | [59] |
| 4f | −26.3 | C6D6 | [62] |
| 4h | −19.6 | C6D6 | [64] |
| 4i | ca. −61.0 | CD3COCD3 | [67] |
| 4j | −29.6 to −33.6 | C6D6 | [68,69] |
| 4k | ca. −42.0 | CD3SOCD3 | [74] |
| PTA | −98.3 | D2O | [77] |
| −101.0 | CDCl3 | [77] | |
| CAP | 46.7 | D2O | [77] |
| 52.8 | CDCl3 | [77] | |
| 47.8 | CDCl3 | [84] | |
| 5a | ca. −87.0 | CDCl3 | [85] |
| 5c | ca. −55.0 | CD3SOCD3 | [91] |
| 5d | −77.9 | CDCl3 | [92] |
| 6ab | −26.5 | C6D6 | [95] |
| 6b | −25.9 | CDCl3 | [96] |
| 6c | −27.4 and −41.5 (JPP 4 Hz) | CDCl3 | [76] |
| 6d | −27.3 | CDCl3 | [97] |
| 6e | −28.0 | CD3SOCD3 | [98] |
| 6f | −27.7 | CDCl3 | [101] |
| 6h | −19.7 | CDCl3 | [103] |
| 6j | −25.3 | CDCl3 | [106] |
| 6k | 30.2 and 36.7 | - | [107] |
| 6l | ca. −34.5 | CDCl3 | [108,109] |
| 6nc | −28.0 | CDCl3 | [112] |
| 6p | 29.5 | C6D6 | [115] |
| 6qc | −26.0 | C6D6 | [116] |
| 6r | −22.1 to −28.1 | CDCl3 | [120] |
| 7ad | 67.0 and −21.7 | CDCl3 | [131] |
| 7b | 79.6 and 7.0 (JPP ~101 Hz) | CDCl3 | [132] |
| 7de | 47.3 | CDCl3 | [48] |
| 8c | 22.3 | C6D6 | [139] |
| 8d | ca. −19.0 | CDCl3 | [140] |
| 8e | −0.4 | C6D6 | [141] |
| 8hf | 25.4 | CDCl3 | [146] |
| 8kg | −7.0 and −19.6 | CDCl3 | [152] |
| 8lh | −52.8 | CDCl3 | [153] |
| 8m | −20.7 and −21.5 | CDCl3 | [154] |
| 9ac | −25.8 and −26.6 | C6D6 | [158] |
| 9ci | −33.5 | CDCl3 | [164] |
| 10a | −22.3 and (JPP 121 Hz) | CDCl3 | [177] |
| 10b | 11.8 | C6D6 | [178] |
| 10e | −14.3 | d8-THF | [186] |
| 10f | −4.9 | CDCl3 | [188] |
| 10h | −14.5 | CDCl3 | [192] |
12. Catalysis
13. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geeson, M.B.; Cummins, C.C. Let’s make white phosphorus obsolete. ACS Cent. Sci. 2020, 6, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Xin, T.; Geeson, M.B.; Cummins, C.C. Sustainable production of reduced phosphorus compounds: Mechanochemical hydride phosphorylation using condensed phosphates as a route to phosphite. ACS Cent. Sci. 2022, 8, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; Cammarata, J.; Schimpf, M.; Wolf, R. Synthesis of monophosphines directly from white phosphorus. Nat. Chem. 2021, 13, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Rothfelder, R.; Streitferdt, V.; Lennert, U.; Cammarata, J.; Scott, D.J.; Zeitler, K.; Gschwind, R.M.; Wolf, R. Photocatalytic arylation of P4 and PH3: Reaction development through mechanistic insight. Angew. Chem. Int. Ed. 2021, 60, 24650–24658. [Google Scholar] [CrossRef] [PubMed]
- Munzeiwa, W.A.; Omondi, B.; Nyamori, V.O. Architecture and synthesis of P,N-heterocyclic phosphine ligands. Beilstein J. Org. Chem. 2020, 16, 362–383. [Google Scholar] [CrossRef]
- Moiseev, D.V.; James, B.R. Phospha-Mannich reactions of PH3 and its analogs. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 277–326. [Google Scholar] [CrossRef]
- Moiseev, D.V.; James, B.R. Phospha-Mannich reactions of RPH2, R2PH and R3P. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 327–391. [Google Scholar] [CrossRef]
- Moiseev, D.V.; James, B.R. Syntheses and rearrangements of tris(hydroxymethyl)phosphine and tetrakis(hydroxymethyl)phosphonium salts. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 687–712. [Google Scholar] [CrossRef]
- Homma, Y.; Fukuda, K.; Iwasawa, N.; Takaya, J. Ruthenium-catalyzed regio- and site-selective ortho C–H borylation of phenol derivatives. Chem. Commun. 2020, 56, 10710–10713. [Google Scholar] [CrossRef]
- Sojka, M.; Tousek, J.; Badri, Z.; Foroutan-Nejad, C.; Necas, M. Bifurcated hydrogen bonds in platinum(II) complexes with phosphinoamine ligands. Polyhedron 2019, 170, 593–601. [Google Scholar] [CrossRef]
- Aucott, S.M.; Slawin, A.M.Z.; Woollins, J.D. The co-ordination chemistry of 2-(diphenylphosphinoamino)pyridine. J. Chem. Soc. Dalton Trans. 2000, 15, 2559–2575. [Google Scholar] [CrossRef]
- Pandey, M.K.; Mague, J.T.; Balakrishna, M.S. Sterically demanding phosphines with 2,6-dibenzhydryl-4-methylphenyl core: Synthesis of RuII, PdII, and PtII Complexes, and structural and catalytic studies. Inorg. Chem. 2018, 57, 7468–7480. [Google Scholar] [CrossRef]
- Groves, L.M.; Ward, B.D.; Newman, P.D.; Horton, P.N.; Coles, S.J.; Pope, S.J.A. Synthesis and characterisation of fluorescent aminophosphines and their coordination to gold(I). Dalton Trans. 2018, 47, 9324–9333. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, X.; Hu, J.; Wang, P.; Wang, S.; Alhumade, H.; Lei, A. Electrochemical oxidative N–H/P–H cross-coupling with H2 evolution towards the synthesis of tertiary phosphines. Chem. Sci. 2022, 13, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- He, L.-P.; Chen, T.; Gong, D.; Lai, Z.; Huang, K.-W. Enhanced reactivities toward amines by introducing an imine arm to the pincer ligand: Direct coupling of two amines to form an imine without oxidant. Organometallics 2012, 31, 5208–5211. [Google Scholar] [CrossRef]
- Li, H.; Goncalves, T.P.; Lupp, D.; Huang, K.-W. PN3(P)-pincer complexes: Cooperative catalysis and beyond. ACS Catal. 2019, 9, 1619–1629. [Google Scholar] [CrossRef]
- Aloisi, A.; Crochet, E.; Nicolas, E.; Berthet, J.-C.; Lescott, C.; Thuéry, P.; Cantat, T. Copper-ligand cooperativity in H2 activation enables the synthesis of copper hydride complexes. Organometallics 2021, 40, 2064–2069. [Google Scholar] [CrossRef]
- Xin, X.; Zhu, C. Isolation of heterometallic cerium(III) complexes with a multidentate nitrogen-phosphorus ligand. Dalton Trans. 2020, 49, 603–607. [Google Scholar] [CrossRef]
- Plajer, A.J.; Zhu, J.; Pröhm, P.; Rizzuto, F.J.; Keyser, U.F.; Wright, D.S. Conformational control in main group phosphazane anion receptors and transporters. J. Am. Chem. Soc. 2020, 142, 1029–1037. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Q.; Xie, Z.; Su, W.; Zhu, J.; Zhu, C. An unprecedented Ga/P frustrated Lewis pair: Synthesis, characterization, and reactivity. Chem. Eur. J. 2019, 25, 14295–14299. [Google Scholar] [CrossRef]
- Shi, K.; Douair, I.; Feng, G.; Wang, P.; Zhao, Y.; Maron, L.; Zhu, C. Heterometallic clusters with multiple rare earth metal–transition metal bonding. J. Am. Chem. Soc. 2021, 143, 5998–6005. [Google Scholar] [CrossRef]
- Thammavongsy, Z.; Cunningham, D.W.; Sutthirat, N.; Eisenhart, R.J.; Ziller, J.W.; Yang, J.Y. Adaptable ligand donor strength: Tracking transannular bond interactions in tris(2-pyridylmethyl)-azaphosphatrane (TPAP). Dalton Trans. 2018, 47, 14101–14110. [Google Scholar] [CrossRef]
- Thammavongsy, Z.; Khosrowabadi Kotyk, J.F.; Tsay, C.; Yang, J.Y. Flexibility is key: Synthesis of a tripyridylamine (TPA) congener with a phosphorus apical donor and coordination to cobalt(II). Inorg. Chem. 2015, 54, 11505–11510. [Google Scholar] [CrossRef]
- Kumar, C.A.; Panda, T.K. Recent development of aminophosphine chalcogenides and boranes as ligands in s-block metal chemistry. Phosphorus Sulfur Silicon 2017, 192, 1084–1101. [Google Scholar] [CrossRef]
- Aguirre, P.A.; Lagos, C.A.; Moya, S.A.; Zúñiga, C.; Vera-Oyarce, C.; Sola, E.; Peris, G.; Bayón, J.C. Methoxycarbonylation of olefins catalyzed by palladium complexes bearing P,N-donor ligands. Dalton Trans. 2007, 46, 5419–5426. [Google Scholar] [CrossRef]
- Kephart, J.A.; Mitchell, B.S.; Chirila, A.; Anderton, K.J.; Rogers, D.; Kaminsky, W.; Velian, A. Atomically defined nanopropeller Fe3Co6Se8(Ph2PNTol)6: Functional model for the electronic metal–support interaction effect and high catalytic activity for carbodiimide formation. J. Am. Chem. Soc. 2019, 141, 19605–19610. [Google Scholar]
- Hsu, C.-W.; Rathnayaka, S.C.; Islam, S.M.; MacMillan, S.N.; Mankad, N.P. N2O reductase activity of a [Cu4S] cluster in the 4CuI redox state modulated by hydrogen bond donors and proton relays in the secondary coordination sphere. Angew. Chem. Int. Ed. 2020, 59, 627–631. [Google Scholar] [CrossRef]
- Yan, L.-L.; Yao, L.-Y.; Yam, V.W.-W. Concentration- and solvation-induced reversible structural transformation and assembly of polynuclear gold(I) sulfido complexes. J. Am. Chem. Soc. 2020, 142, 11560–11568. [Google Scholar] [CrossRef]
- Böttcher, H.-C.; Heinemann, J. Synthesis and molecular structure of [Ru3(CO)10(μ-dppa)] (dppa = Ph2PN(H)PPh2) provided by its dioxane solvate. Z. Anorg. Allg. Chem. 2020, 646, 1787–1789. [Google Scholar] [CrossRef]
- Johnson, B.J.; Lindeman, S.V.; Mankad, N.P. Assembly, structure, and reactivity of Cu4S and Cu3S models for the nitrous oxide reductase active site, Cuz*. Inorg. Chem. 2014, 53, 10611–10619. [Google Scholar] [CrossRef]
- Wang, F.T.; Najdzionek, J.; Leneker, K.L.; Wasserman, H.; Braitsch, D.M. A facile synthesis of imidotetraphenyldiphosphinic acids. Synth. React. Inorg. Met. Org. Chem. 1978, 8, 119–125. [Google Scholar] [CrossRef]
- Bollman, A.; Blann, K.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; McGuinness, D.S.; Morgan, D.H.; Neveling, A.; Otto, S.; et al. Ethylene tetramerization: A new route to produce 1-octene in exceptionally high selectivities. J. Am. Chem. Soc. 2004, 126, 14712–14713. [Google Scholar] [CrossRef]
- Kuhlmann, S.; Blann, K.; Bollmann, A.; Dixon, J.T.; Killian, E.; Maumela, M.C.; Maumela, H.; Morgan, D.H.; Prétoruis, M.; Taccardi, N.; et al. N-substituted diphosphinoamines: Toward rational ligand design for the efficient tetramerization of ethylene. J. Catal. 2007, 245, 279–284. [Google Scholar] [CrossRef]
- Blann, K.; Bollmann, A.; de Bod, H.; Dixon, J.T.; Killian, E.; Nongodlwana, P.; Maumela, M.C.; Maumela, H.; McConnell, A.E.; Morgan, D.H.; et al. Ethylene tetramerisation: Subtle effects exhibited by N-substituted diphosphinoamine ligands. J. Catal. 2007, 249, 244–249. [Google Scholar] [CrossRef]
- Xiao, Z.; Johnson, A.; Singh, K.; Suntharalingam, K. The discrete breast cancer stem cell mammosphere activity of group 10-bis(azadiphosphine) metal complexes. Angew. Chem. Int. Ed. 2021, 60, 6704–6709. [Google Scholar] [CrossRef]
- Song, L.-C.; Zhang, L.-D.; Zhang, W.-W.; Liu, B.-B. Heterodinuclear Ni/M (M = Mo, W) complexes relevant to the active site of [NiFe]-hydrogenases: Synthesis, characterization, and electrocatalytic H2 evolution. Organometallics 2018, 37, 1948–1957. [Google Scholar] [CrossRef]
- Zhao, P.-H.; Ma, Z.-Y.; Hu, M.-Y.; He, J.; Wang, Y.-Z.; Jing, X.-B.; Chen, H.-Y.; Wang, Z.; Li, Y.-L. PNP-Chelated and -bridged diiron dithiolate complexes Fe2(μ-pdt)(CO)4{(Ph2P)2NR} together with related monophosphine complexes for the [2Fe]H subsite of [FeFe]-hydrogenases: Preparation, structure, and electrocatalysis. Organometallics 2018, 37, 1280–1290. [Google Scholar] [CrossRef]
- Kathewad, N.; Kumar, N.; Dasgupta, R.; Ghosh, M.; Pal, S.; Khan, S. The syntheses and photophysical properties of PNP-based Au(I) complexes with strong intramolecular Au…Au interactions. Dalton Trans. 2019, 48, 7274–7280. [Google Scholar] [CrossRef]
- Pal, S.; Kathewad, N.; Pant, R.; Khan, S. Synthesis, characterization, and luminescence studies of gold(I) complexes with PNP- and PNB-based ligand systems. Inorg. Chem. 2015, 54, 10172–10183. [Google Scholar] [CrossRef]
- Kathewad, N.; Pal, S.; Kumawat, R.L.; Ehesan Ali, M.; Khan, S. Synthetic diversity and luminescence properties of ArN(PPh2)2-based copper(I) complexes. Eur. J. Inorg. Chem. 2018, 2518–2523. [Google Scholar] [CrossRef]
- Gaw, K.G.; Smith, M.B.; Wright, J.B.; Slawin, A.M.Z.; Coles, S.J.; Hursthouse, M.B.; Tizzard, G.J. Square-planar metal(II) complexes containing ester functionalised bis(phosphino)amines: Mild P–N methanolysis and Carene–H cyclometallation. J. Organomet. Chem. 2012, 699, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Höhne, M.; Joksch, M.; Konieczny, K.; Müller, B.H.; Spannenberg, A.; Peulecke, N.; Rosenthal, U. Selective reductions of N,N-bis{chloro(aryl)-phosphino}-amines yielding three-, five-, six-, and eight-membered cyclic azaphosphanes. Chem. Eur. J. 2017, 23, 4298–4309. [Google Scholar] [CrossRef] [PubMed]
- Dickie, D.A.; Barker, M.T.; Land, M.A.; Hughes, K.E.; Clyburne, J.A.C.; Kemp, R.A. Rapid, reversible, solid–gas and solution-phase insertion of CO2 into In–P bonds. Inorg. Chem. 2015, 54, 11121–11126. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Kumar, S.; Mague, J.T.; Balakrishna, M.S. A hybrid terpyridine-based bis(diphenylphosphino)amine ligand, terpy-C6H4N(PPh2)2: Synthesis, coordination chemistry and photoluminescence studies. Dalton Trans. 2016, 45, 18434–18437. [Google Scholar] [CrossRef]
- Todisco, S.; Mastrorilli, P.; Latronico, M.; Gallo, V.; Englert, U.; Re, N.; Creati, F.; Braunstein, P. Sulfur-assisted phenyl migration from phosphorus to platinum in PtW2 and PtMo2 clusters containing thioether-functionalized short-bite ligands of the bis(diphenylphosphanyl)amine-type. Inorg. Chem. 2015, 54, 4777–4798. [Google Scholar] [CrossRef]
- Gallo, V.; Mastrorilli, P.; Nobile, C.F.; Braunstein, P.; Englert, U. Chelating versus bridging bonding modes of N-substituted bis(diphenylphosphanyl)amine ligands in Pt complexes and Co2Pt clusters. Dalton Trans. 2006, 2342–2349. [Google Scholar] [CrossRef]
- Kama, D.V.; Frei, A.; Brink, A.; Braband, H.; Alberto, R.; Roodt, A. New approach for the synthesis of water soluble fac-[MI(CO)3]+ bis(diarylphosphino)alkylamine complexes (M = 99Tc, Re). Dalton Trans. 2021, 50, 17506–17514. [Google Scholar] [CrossRef]
- Bowen, L.E.; Charernsuk, M.; Hey, T.W.; McMullin, C.L.; Orpen, A.G.; Wass, D.F. Ligand effects in chromium diphosphine catalysed olefin co-trimerisation and diene trimerisation. Dalton Trans. 2010, 39, 560–567. [Google Scholar] [CrossRef]
- Haddow, M.F.; Jaltai, J.; Hanton, M.; Pringle, P.G.; Rush, L.E.; Sparkes, H.A.; Woodall, C.H. Aminophobanes: Hydrolytic stability, tautomerism and application in Cr-catalysed ethene oligomerisation. Dalton Trans. 2016, 45, 2294–2307. [Google Scholar] [CrossRef]
- Makume, B.F.; Holzapfel, C.W.; Maumela, M.C.; Willemse, J.A.; van den Berg, J.A. Ethylene tetramerisation: A structure-selectivity correlation. ChemPlusChem 2020, 85, 2308–2315. [Google Scholar] [CrossRef]
- Maumela, M.C.; Blann, K.; de Bod, H.; Dixon, J.T.; Gabrielli, W.F.; Williams, D.B.G. Efficient synthesis of novel N-substituted bulky diphosphinoamines. Synthesis 2007, 2007, 3863–3867. [Google Scholar] [CrossRef]
- Fei, Z.; Biricik, N.; Zhao, D.; Scopelliti, R.; Dyson, P.J. Transformation between diphosphinoamines and iminobiphosphines: A reversible P–N–P↔N = P–P rearrangement triggered by protonation/deprotonation. Inorg. Chem. 2004, 43, 2228–2230. [Google Scholar] [CrossRef]
- Lifschitz, A.M.; Hirscher, N.A.; Lee, H.B.; Buss, J.A.; Agapie, T. Ethylene tetramerization catalysis: Effects of aluminium-induced isomerization of PNP to PPN ligands. Organometallics 2017, 36, 1640–1648. [Google Scholar] [CrossRef]
- Cloete, N.; Visser, H.G.; Engelbrecht, I.; Overett, M.J.; Gabrielli, W.F.; Roodt, A. Ethylene tri- and tetramerization: A steric parameter selectivity switch from X-ray crystallography and computational analysis. Inorg. Chem. 2013, 52, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; McCarthy, S.M.; Lai, T.Y.; Yennawar, H.P.; Radosevich, A.T. Reversible intermolecular E–H oxidative addition to a geometrically deformed and structurally dynamic phosphorous triamide. J. Am. Chem. Soc. 2014, 136, 17634–17644. [Google Scholar] [CrossRef]
- Cao, Y.; Napoline, J.W.; Bacsa, J.; Pollet, P.; Soper, J.D.; Sadighi, J.P. Synthesis of an azaphosphatriptycene and its rhodium carbonyl complex. Organometallics 2019, 38, 1868–1871. [Google Scholar] [CrossRef]
- Payet, E.; Auffrant, A.; Le Goff, X.F.; Le Floch, P. Phosphine- and thiophosphorane-amine ligands: Lithiation and coordination to Rh(I). J. Organomet. Chem. 2010, 695, 1499–1506. [Google Scholar] [CrossRef]
- Kumar, S.; Mondal, D.; Balakrishna, M.S. Diverse architectures and luminescence properties of Group 11 complexes containing pyrimidine-based phosphine, N-((diphenylphosphine)methyl)pyrimidin-2-amine. ACS Omega 2018, 3, 16601–16614. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Zhang, M.; Chen, X.-R.; Lu, C.; Young, D.J.; Ren, Z.-G.; Lang, J.-P. Cobalt(II) and nickel(II) complexes of a PNN type ligand as photoenhanced electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. 2020, 59, 1038–1045. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Hu, S.; Young, D.J.; Lu, C.; Li, H.-X.; Ren, Z.-G. A photoluminescent Au(I)/Ag(I)/PNN coordination complex for relatively rapid and reversible alcohol sensing. Dalton Trans. 2021, 50, 6773–6777. [Google Scholar] [CrossRef]
- de Almeida, R.F.M.; Santos, T.C.B.; da Silva, L.C.; Suchodolski, J.; Krasowska, A.; Stokowa-Soltys, K.; Puchalska, M.; Starosta, R. NBP derived diphenyl(aminomethyl)phosphane—A new fluorescent dye for imaging of low pH regions and lipid membranes in living cells. Dye. Pigment. 2021, 184, 108771. [Google Scholar] [CrossRef]
- Hazari, A.; Labinger, J.A.; Bercaw, J.E. A versatile ligand platform that supports Lewis acid promoted migratory insertion. Angew. Chem. Int. Ed. 2012, 51, 8268–8271. [Google Scholar] [CrossRef]
- De’Ath, P.; Elsegood, M.R.J.; Halliwell, C.A.G.; Smith, M.B. Mild intramolecular P–C(sp3) bond cleavage in bridging diphosphine complexes of RuII, RhIII, and IrIII. J. Organomet. Chem. 2021, 937, 121704. [Google Scholar] [CrossRef]
- Cui, P.; Xiong, C.; Du, J.; Huang, Z.; Xie, S.; Wang, H.; Zhou, S.; Fang, H.; Wang, S. Heterobimetallic scandium-group 10 metal complexes with LM ⟶ Sc (LM = Ni, Pd, Pt) dative bonds. Dalton Trans. 2020, 49, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Liu, S.; Gagliardi, L.; Young, V.G., Jr.; Lu, C.C. Metal-alane adducts with zero-valent nickel, cobalt, and iron. J. Am. Chem. Soc. 2011, 133, 20724–20727. [Google Scholar] [CrossRef] [PubMed]
- Majoral, J.P.; Zablocka, M.; Caminade, A.-M.; Balczewski, P.; Shi, X.; Mignani, S. Straightforward synthesis of gold nanoparticles by adding water to an engineered small dendrimer. Coord. Chem. Rev. 2018, 358, 80–91. [Google Scholar] [CrossRef]
- Starosta, R.; Florek, M.; Król, J.; Puchalska, M.; Kochel, A. Copper(I) iodide complexes containing new aliphatic aminophosphine ligands and diimines–luminescent properties and antibacterial activity. New J. Chem. 2010, 34, 1441–1449. [Google Scholar] [CrossRef]
- Raturi, R.; Lefebvre, J.; Leznoff, D.B.; McGarvey, B.R.; Johnson, S.A. A phosphine-mediated through-space exchange coupling pathway for unpaired electrons in a heterobimetallic lanthanide–transition metal complex. Chem. Eur. J. 2008, 14, 721–730. [Google Scholar] [CrossRef]
- Han, H.; Elsmaili, M.; Johnson, S.A. Diligating tripodal amido-phosphine ligands: The effect of a proximal antipodal early transition metal on phosphine donor ability in a building block for heterometallic complexes. Inorg. Chem. 2006, 45, 7435–7445. [Google Scholar] [CrossRef]
- Han, H.; Johnson, S.A. Bridged dinuclear tripodal tris(amido)phosphane complexes of titanium and zirconium as diligating building blocks for organometallic polymers. Eur. J. Inorg. Chem. 2008, 471–482. [Google Scholar] [CrossRef]
- Han, H.; Johnson, S.A. Ligand design for the assembly of polynuclear complexes: Synthesis and structures of trinuclear and tetranuclear aluminum alkyl complexes bearing tripodal diamidoselenophosphinito ligands and a comparison to related tripodal triamidophosphine complexes. Organometallics 2006, 25, 5594–5602. [Google Scholar] [CrossRef]
- Keen, A.L.; Doster, M.; Han, H.; Johnson, S.A. Facile assembly of a Cu9 amido complex: A new tripodal ligand design that promotes transition metal cluster formation. Chem. Commun. 2006, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Hatnean, J.A.; Raturi, R.; Lefebvre, J.; Leznoff, D.B.; Lawes, G.; Johnson, S.A. Assembly of triangular trimetallic complexes by triamidophosphine ligands: Spin-frustrated Mn2+ plaquettes and diamagnetic Mg2+ analogues with a combined through-space, through-bond pathway for 31P-31P spin–spin coupling. J. Am. Chem. Soc. 2006, 128, 14992–14999. [Google Scholar] [CrossRef] [PubMed]
- Carpenter-Warren, C.L.; Cunnington, M.; Elsegood, M.R.J.; Kenny, A.; Hill, A.R.; Miles, C.R.; Smith, M.B. Synthesis, metal coordination and structural studies of trisubstituted P{CH2-1-N(H)naphthyl}3 ligands. Inorg. Chim. Acta 2017, 462, 289–297. [Google Scholar] [CrossRef]
- Cui, P.; Huang, X.; Du, J.; Huang, Z. P–C bond cleavage induced Ni(II) complexes bearing rare-earth-metal-based metalloligand and reactivities towards isonitrile, nitrile, and epoxide. Inorg. Chem. 2021, 60, 3249–3258. [Google Scholar] [CrossRef]
- Brown, G.M.; Elsegood, M.R.J.; Lake, A.J.; Sanchez-Ballester, N.M.; Smith, M.B.; Varley, T.S.; Blann, K. Mononuclear and heterodinuclear metal complexes of nonsymmetric ditertiary phosphanes derived from R2PCH2OH. Eur. J. Inorg. Chem. 2007, 1405–1414. [Google Scholar] [CrossRef]
- Guerriero, A.; Gonsalvi, L. From traditional PTA to novel CAP: A comparison between two adamantane cage-type aminophosphines. Inorg. Chim. Acta 2021, 518, 120251. [Google Scholar] [CrossRef]
- Lanorio, J.P.; Mebi, C.A.; Frost, B.J. The synthesis, structure and H/D exchange reactions of water-soluble half-sandwich ruthenium(II) hydrides of indenyl and dihydropentalenyl. Organometallics 2019, 38, 2031–2041. [Google Scholar] [CrossRef]
- Battistin, F.; Balducci, G.; Milani, B.; Alessio, E. Water-soluble ruthenium(II) carbonyls with 1,3,5-triaza-7-phosphoadamantane. Inorg. Chem. 2018, 57, 6991–7005. [Google Scholar] [CrossRef]
- Mager, N.; Robeyns, K.; Hermans, S. Synthesis of water-soluble ruthenium clusters by reaction with PTA (1,3,5-triaza-7-phosphaadamantane). J. Organomet. Chem. 2015, 794, 48–58. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Imberti, S.; Bernasconi, L.; Jadagayeva, N.; Scalambra, F.; Romerosa, A. Study of the interaction of water with the aqua-soluble dimeric complex [RuCp(PTA)2–μ-CN–1κC:2κ2N-RuCp(PTA)2](CF3SO3) (PTA = 1,3,5-triaza-7-phosphaadamantane) by neutron and X-ray diffraction in solution. Chem. Commun. 2014, 50, 11587–11590. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Britvin, S.N.; Lotnyk, A. Water-soluble phosphine capable of dissolving elemental gold: The missing link between 1,3,5-triaza-7-phosphaadamantane (PTA) and Verkade’s ephemeral ligand. J. Am. Chem. Soc. 2015, 137, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, T.; Voloshkin, V.A.; Martynova, E.; Vanden Broeck, S.M.P.; Beliš, M.; Cazin, C.S.J.; Nolan, S.P. Synthesis and catalytic activity of palladium complexes bearing N-heterocyclic carbenes (NHCs) and 1,4,7-triaza-9-phosphatricyclo[5.3.2.1]tridecane (CAP) ligands. Dalton Trans. 2021, 50, 9491–9499. [Google Scholar] [CrossRef] [PubMed]
- Enow, R.A.E.; Lee, W.-C.; Cournoyer, T.D.; Sunderland, T.L.; Frost, B.J. Unusual water-soluble imino phosphine ligand: Enamine and imine derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA). Inorg. Chem. 2018, 57, 9142–9152. [Google Scholar] [CrossRef]
- Sears, J.M.; Lee, W.-C.; Frost, B.J. Water soluble diphosphine ligands based on 1,3,5-triaza-7-phosphaadamantane (PTA-PR2): Synthesis, coordination chemistry, and ruthenium catalyzed nitrile hydration. Inorg. Chim. Acta 2015, 431, 248–257. [Google Scholar] [CrossRef]
- Krogstad, D.A.; Guerriero, A.; Ienco, A.; Manca, G.; Peruzzini, M.; Reginato, G.; Gonsalvi, L. Imidazolyl-PTA derivatives as water-soluble (P,N) ligands for ruthenium-catalyzed hydrogenations. Organometallics 2011, 30, 6292–6302. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Blaszczyk, J.; Sieroń, L.; Lielbasiński, P. Enzymatic approach to the synthesis of enantiomerically pure hydroxy derivatives of 1,3,5-triaza-7-phosphaadamantane. J. Org. Chem. 2021, 86, 8556–8562. [Google Scholar] [CrossRef]
- Ramarou, D.S.; Makhubela, B.C.E.; Smith, G.S. Synthesis of Rh(I) alkylated-PTA complexes as catalyst precursors in the aqueous-biphasic hydroformylation of 1-octene. J. Organomet. Chem. 2018, 870, 23–31. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gascón, S.; Rodríguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel gold(I) thiolate derivatives synergistic with 5-fluorouracil as potential selective anticancer agents in colon cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef]
- Ekubo, A.T.; Elsegood, M.R.J.; Lake, A.J.; Smith, M.B. Intramolecular hydrogen-bonded tertiary phosphines as 1,3,5-triaza-7-phosphaadamantane (PTA) analogues. Inorg. Chem. 2009, 48, 2633–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A new amido-phosphane as ligand for copper and silver complexes. Synthesis, characterization and catalytic application for azide–alkyne cycloaddition in glycerol. Dalton Trans. 2021, 50, 6109–6125. [Google Scholar] [CrossRef] [PubMed]
- Battistin, F.; Vidal, A.; Cavigli, P.; Balducci, G.; Iengo, E.; Alessio, E. Orthogonal coordination chemistry of PTA towards Ru(II) and Zn(II) (PTA = 1,3,5-triaza-7-phosphaadamantane) for the construction of 1D and 2D metal-mediated porphyrin networks. Inorg. Chem. 2020, 59, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Bálint, E.; Tajti, A.; Tripolszky, A.; Keglevich, G. Synthesis of platinum, palladium and rhodium complexes of α-aminophosphine ligands. Dalton Trans. 2018, 47, 4755–4778. [Google Scholar] [CrossRef] [PubMed]
- Klemps, C.; Payet, E.; Magna, L.; Saussine, L.; Le Goff, X.F.; Le Floch, P. PCNCP ligands in the chromium-catalyzed oligomerization of ethylene: Tri- versus tetramerization. Chem. Eur. J. 2009, 15, 8259–8268. [Google Scholar] [CrossRef]
- Pandey, M.K.; Kunchur, H.S.; Mondal, D.; Radhakrishna, L.; Kote, B.S.; Balakrishna, M.S. Rare Au…H interactions in gold(I) complexes of bulky phosphines derived from 2,6-dibenzhydryl-4-methylphenyl core. Inorg. Chem. 2020, 59, 3642–3658. [Google Scholar] [CrossRef]
- Cao, B.; Elsegood, M.R.J.; Lastra-Calvo, N.; Smith, M.B. New (aminomethyl)phosphines via selective hydrophosphination and/or phosphorus based Mannich condensation reactions. J. Organomet. Chem. 2017, 853, 159–167. [Google Scholar] [CrossRef]
- Wang, J.-F.; Liu, S.-Y.; Liu, C.-Y.; Ren, Z.-G.; Lang, J.-P. Silver(I) complexes with a P–N hybrid ligand and oxyanions: Synthesis, structures, photocatalysis and photocurrent responses. Dalton Trans. 2016, 45, 9294–9306. [Google Scholar] [CrossRef]
- Jiang, M.-S.; Tao, Y.-H.; Wang, Y.-W.; Lu, C.; Young, D.J.; Lang, J.-P.; Ren, Z.-G. Reversible solid-state phase transitions between Au–P complexes accompanied by switchable fluorescence. Inorg. Chem. 2020, 59, 3072–3078. [Google Scholar] [CrossRef]
- Xu, W.-D.; Yan, J.-J.; Feng, M.-Y.; Li, H.-Y.; Young, D.J.; Ren, Z.-G. A photoluminescent thermometer made from a thermoresponsive tetranuclear gold complex and phosphor N630. Dalton Trans. 2021, 50, 16395–16400. [Google Scholar] [CrossRef]
- Penney, M.K.; Giang, R.; Klausmeyer, K.K. Single, double, and triple silver centers bound by a tetradentate N,P ligand. Polyhedron 2015, 85, 275–283. [Google Scholar] [CrossRef]
- Wang, X.-J.; Gui, L.-C.; Ni, Q.-L.; Liao, Y.-F.; Jiang, X.-F.; Tang, L.-H.; Zhang, Z.; Wu, Q. π-Stacking induced complexes with Z-shape motifs featuring a complementary approach between electron-rich arene diamines and electron-deficient aromatic N-heterocycles. CrystEngComm 2008, 10, 1003–1010. [Google Scholar] [CrossRef]
- Mondal, D.; Sardar, G.; Kabra, D.; Balakrishna, M.S. 2,2′-Bipyridine derived doubly B ← N fused bisphosphine-chalcogenides, [C5H3N(BF2){NCH2P(E)Ph2}]2 (E = O, S, Se): Tuning of structural features and photophysical studies. Dalton Trans. 2022, 51, 6884–6898. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, X.-Y.; Wang, H.-F.; Young, D.J.; Ren, Z.-G.; Lang, J.-P. Novel silver–phosphine coordination polymers incorporating a Wurster’s blue–like radical cation with enhanced photoelectric properties. Chem. Commun. 2019, 55, 6599–6602. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Huang, T.-H.; Wang, X.-J.; Jiang, X.-F.; Ni, Q.-L.; Gui, L.-C.; Fan, Y.-J.; Tan, Y.-L. Synthesis, structural characterization and luminescent properties of a series of Cu(I) complexes based on polyphosphine ligands. Dalton Trans. 2011, 40, 7551–7558. [Google Scholar] [CrossRef]
- Navrátil, M.; Cisařová, I.; Štepnicka, P. Synthesis, coordination and electrochemistry of a ferrocenyl-tagged aminobisphosphane ligand. Eur. J. Inorg. Chem. 2021, 3781–3792. [Google Scholar] [CrossRef]
- Kreienbrink, A.; Lönnecke, P.; Findeisen, M.; Hey-Hawkins, E. Endocyclic P–P bond cleavage in carbaborane-substituted 1,2-diphosphetane: A new route to secondary phosphinocarbaboranes. Chem. Commun. 2012, 48, 9385–9387. [Google Scholar] [CrossRef]
- Edgar, M.; Elsegood, M.R.J.; Liu, P.; Miles, C.R.; Smith, M.B.; Wu, S. Dinuclear palladium(II) and platinum(II) complexes of a readily accessible bicyclic diphosphane. Eur. J. Inorg. Chem. 2022, e202200017. [Google Scholar] [CrossRef]
- Coles, S.J.; Horton, P.N.; Kimber, P.; Klooster, W.T.; Liu, P.; Plasser, F.; Smith, M.B.; Tizzard, G.J. Reversible P–P bond cleavage at an iridium(III) metal centre. Chem. Commun. 2022, 58, 5598–5601. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Hegedüs, L.; Keglevich, G. Microwave-assisted synthesis of N,N-bis(phosphinoylmethyl)amines and N,N,N-tris(phosphinoylmethyl)amines bearing different substituents on the phosphorus atoms. Belstein J. Org. Chem. 2019, 15, 469–473. [Google Scholar] [CrossRef]
- Phanopoulos, A.; White, A.J.P.; Long, N.J.; Miller, P.W. Insight into the stereoelectronic parameters of N-triphos ligands via coordination to tungsten(0). Dalton Trans. 2016, 45, 5536–5548. [Google Scholar] [CrossRef]
- Miller, P.W.; White, A.J.P. The preparation of multimetallic complexes using sterically bulky N-centred tripodal dialkyl phosphino ligands. J. Organomet. Chem. 2010, 695, 1138–1145. [Google Scholar] [CrossRef]
- Phanopoulos, A.; Brown, N.J.; White, A.J.P.; Long, N.J.; Miller, P.W. Synthesis, characterization, and reactivity of ruthenium hydride complexes of N-centred triphosphine ligands. Inorg. Chem. 2014, 53, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Fillol, J.L.; Kruckenberg, A.; Scherl, P.; Wadepohl, H.; Gade, L.H. Stitching phospholanes together piece by piece: New modular di- and tridentate stereodirecting ligands. Chem. Eur. J. 2011, 17, 14047–14062. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Yamashita, M.; Nozaki, K. Syntheses of PBP pincer iridium complexes: A supporting boryl ligand. J. Am. Chem. Soc. 2009, 131, 9201–9203. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; McQueen, C.M.A. Dihydroperimidine-derived N-heterocyclic pincer carbene complexes via double C–H activation. Organometallics 2012, 31, 8051–8054. [Google Scholar] [CrossRef]
- McQueen, C.M.A.; Hill, A.F.; Ma, C.; Ward, J.S. Ruthenium and osmium complexes of dihydroperimidine-based N-heterocyclic carbene pincer ligands. Dalton Trans. 2015, 44, 20376–20385. [Google Scholar] [CrossRef]
- De’Ath, P.; Elsegood, M.R.J.; Sanchez-Ballester, N.M.; Smith, M.B. Low-dimensional architectures in isomeric cis-PtCl2{Ph2PCH2N(Ar)CH2PPh2} complexes using regioselective-N(Aryl)-group manipulation. Molecules 2021, 26, 6809. [Google Scholar] [CrossRef]
- Smith, M.B.; Dale, S.H.; Coles, S.J.; Gelbrich, T.; Hursthouse, M.B.; Light, M.E.; Horton, P.N. Hydrogen bonded supramolecular assemblies based on neutral square-planar palladium(II) complexes. CrystEngComm 2007, 9, 165–175. [Google Scholar] [CrossRef]
- Smith, M.B.; Dale, S.H.; Coles, S.J.; Gelbrich, T.; Hursthouse, M.B.; Light, M.E. Isomeric dinuclear gold(I) complexes with highly functionalised ditertiary phosphines: Self-assembly of dimers, rings and 1-D polymeric chains. CrystEngComm 2006, 8, 140–149. [Google Scholar] [CrossRef]
- Elsegood, M.R.J.; Smith, M.B.; Staniland, P.M. Neutral molecular Pd6 hexagons using κ3-P2O-terdentate ligands. Inorg. Chem. 2006, 45, 6761–6770. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.E.; Durran, S.E.; Elsegood, M.R.J.; Smith, M.B.; Staniland, P.M.; Talib, S.; Dale, S.H. Supramolecular chemistry of half-sandwich organometallic building blocks based on RuCl2(p-cymene)Ph2PCH2Y. J. Organomet. Chem. 2006, 691, 4829–4842. [Google Scholar] [CrossRef]
- Plotek, M.; Starosta, R.; Komarnicka, U.K.; Skórska-Stania, A.; Stochel, G.; Kyziol, A.; Jezowska-Bojczuk, M. Unexpected formation of [Ru(η5-C5H5)(PH{CH2N-(CH2CH2)2O}2)(PPh3)2]BF4—The first “piano-stool” ruthenium complex bearing a secondary aminomethylphosphane ligand. RSC Adv. 2015, 5, 2952–2955. [Google Scholar] [CrossRef]
- Cooper, S.M.; White, A.J.P.; Eykyn, T.R.; Ma, M.T.; Miller, P.W.; Long, N.J. N-Centered tripodal phosphine Re(V) and Tc(V) oxo complexes: Revisiting a [3 + 2] mixed-ligand approach. Inorg. Chem. 2022, 61, 8000–8014. [Google Scholar] [CrossRef]
- Chambers, G.M.; Johnson, S.I.; Raugei, S.; Bullock, R.M. Anion control of tautomeric equilibria: Fe–H vs. N–H influenced by NH…F hydrogen bonding. Chem. Sci. 2019, 10, 1410–1418. [Google Scholar] [CrossRef]
- Ezzaher, S.; Capon, J.-F.; Gloaguen, F.; Pétillon, F.Y.; Schollhammer, P.; Talarmin, J. Influence of a pendant amine in the second coordination sphere on proton transfer at a dissymmetrically disubstituted diiron system related to the [2Fe]H subsite of [FeFe]H2ase. Inorg. Chem. 2009, 48, 2–4. [Google Scholar] [CrossRef]
- Zhang, S.; Bullock, R.M. Molybdenum hydride and dihydride complexes bearing diphosphine ligands with a pendant amine: Formation of complexes with bound amines. Inorg. Chem. 2015, 54, 6397–6409. [Google Scholar] [CrossRef]
- Walsh, A.P.; Laureanti, J.A.; Katipamula, S.; Chambers, G.M.; Priyadarshani, N.; Lense, S.; Bays, J.T.; Linehan, J.C.; Shaw, W.J. Evaluating the impacts of amino acids in the second and outer coordination spheres of Rh-bis(diphosphine) complexes for CO2 hydrogenation. Faraday Discuss. 2019, 215, 123–140. [Google Scholar] [CrossRef]
- Laureanti, J.A.; Su, Q.; Shaw, W.J. A protein scaffold enables hydrogen evolution for a Ni-bisdiphosphine complex. Dalton Trans. 2021, 50, 15754–15759. [Google Scholar] [CrossRef]
- Zheng, Y.; Nie, X.; Long, Y.; Ji, L.; Fu, H.; Zheng, X.; Chen, H.; Li, R. Ruthenium-catalysed synthesis of N-substituted lactams by acceptorless dehydrogenative coupling of diols with primary amines. Chem. Commun. 2019, 55, 12384–12387. [Google Scholar] [CrossRef]
- Blann, K.; Bollmann, A.; Brown, G.M.; Dixon, J.T.; Elsegood, M.R.J.; Raw, C.R.; Smith, M.B.; Tenza, K.; Willemse, J.A.; Zweni, P. Ethylene oligomerisation chromium catalysts with unsymmetrical PCNP ligands. Dalton Trans. 2021, 50, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Brill, M.; Rominger, F.; Hofmann, P. 1,2-Bis(di-tert-butylphosphino)imidazole (dtbpi): A versatile imidazole-based, rigid, bulky bisphosphine ligand for transition metals. Organometallics 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Wang, Z.; Zhao, X.; Mi, P.; Zhang, J. Ethylene tri-/tetramerization catalysts supported by diphosphinoindole ligands. J. Organomet. Chem. 2022, 958, 122175. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef]
- Kar, S.; Milstein, D. Sustainable catalysis with fluxional acridine-based PNP pincer complexes. Chem. Commun. 2022, 58, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, P.; López, P.; García-Orduña, P.; Lahoz, F.J.; Polo, V.; Casado, M.A. Amido complexes of iridium with a PNP pincer ligand: Reactivity towards alkynes and hydroamination catalysis. Organometallics 2018, 37, 2618–2629. [Google Scholar] [CrossRef]
- Kirlin, F.L.; Borden, O.J.; Head, M.C.; Kelly, S.E.; Chianese, A.R. Epoxide hydrogenolysis catalyzed by ruthenium PNN and PNP pincer complexes. Organometallics 2022, 41, 1025–1033. [Google Scholar] [CrossRef]
- Abogosh, A.K.; Alghanem, M.K.; Ahmad, S.; Al-Asmari, A.; As Sobeai, H.M.; Sulaiman, A.A.A.; Fettouchi, M.; Popoola, S.A.; Alhoshani, A.; Isab, A.A. A novel cyclic dinuclear gold(I) complex induces anticancer activity via an oxidative stress-mediated intrinsic apoptotic pathway in MDA-MB-231 cancer cells. Dalton Trans. 2022, 51, 2760–2769. [Google Scholar] [CrossRef]
- Meiners, J.; Friedrich, A.; Herdtweek, E.; Schneider, S. Facile double C–H activation of tetrahydrofuran by an iridium PNP pincer complex. Organometallics 2009, 28, 6331–6338. [Google Scholar] [CrossRef]
- Salvarese, N.; Refosco, F.; Seraglia, R.; Roverso, M.; Dolmella, A.; Bolzati, C. Synthesis and characterization of rhenium(III) complexes with (Ph2PCH2CH2)2NR diphosphinoamine ligands. Dalton Trans. 2017, 46, 9180–9191. [Google Scholar] [CrossRef]
- Curley, J.B.; Hert, C.; Bernskoetter, W.H.; Hazari, N.; Mercado, B.Q. Control of catalyst isomers using an N-phenyl-substituted RN(CH2CH2PiPr2)2 pincer ligand in CO2 hydrogenation and formic acid dehydrogenation. Inorg. Chem. 2022, 61, 643–656. [Google Scholar] [CrossRef]
- Curley, J.B.; Townsend, T.M.; Bernskoetter, W.H.; Hazari, N.; Mercado, B.Q. Iron, cobalt, and nickel complexes supported by a iPrPNPhP pincer ligand. Organometallics 2022, 41, 301–312. [Google Scholar] [CrossRef]
- Lapointe, S.; Khaskin, E.; Fayzullin, R.R.; Khusnutdinova, J.R. Nickel(II) complexes with electron-rich, sterically hindered PNP pincer ligands enable uncommon modes of ligand dearomatization. Organometallics 2019, 38, 4433–4447. [Google Scholar] [CrossRef]
- Deolka, S.; Faysullin, R.R.; Khaskin, E. Bulky PNP ligands blocking metal-ligand cooperation allow for isolation of Ru(0), and lead to catalytically active Ru complexes in acceptorless alcohol dehydrogenation. Chem. Eur. J. 2022, 28, e202103778. [Google Scholar] [CrossRef]
- Deolka, S.; Tarannam, N.; Fayzullin, R.R.; Kozuch, S.; Khaskin, E. Unusual rearrangement of modified PNP ligand based Ru complexes relevant to alcohol dehydrogenation catalysis. Chem. Commun. 2019, 55, 11350–11353. [Google Scholar] [CrossRef] [PubMed]
- Reiner, B.R.; Mucha, N.T.; Rothstein, A.; Temme, J.S.; Duan, P.; Schmidt-Rohr, K.; Foxman, B.M.; Wade, C.R. Zirconium metal–organic frameworks assembled from Pd and Pt PNNNP pincer complexes: Synthesis, postsynthetic modification, and Lewis acid catalysis. Inorg. Chem. 2018, 57, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Das, U.K.; Chakraborty, S.; Diskin-Posner, Y.; Milstein, D. Direct conversion of alcohols into alkenes by dehydrogenative coupling with hydrazine/hydrazone catalyzed by manganese. Angew. Chem. Int. Ed. 2018, 57, 13444–13448. [Google Scholar] [CrossRef]
- Kaithal, A.; Hölscher, M.; Leitner, W. Catalytic hydrogenation of cyclic carbonates using manganese complexes. Angew. Chem. Int. Ed. 2018, 57, 13449–13453. [Google Scholar] [CrossRef]
- Ryabchuk, P.; Stier, K.; Junge, K.; Checinski, M.P.; Beller, M. Molecularly defined manganese catalyst for low-temperature hydrogenation of carbon monoxide to methanol. J. Am. Chem. Soc. 2019, 141, 16923–16929. [Google Scholar] [CrossRef]
- Kaithal, A.; Hölscher, M.; Leitner, W. Carbon monoxide and hydrogen (syngas) as a C1-building block for selective catalytic methylation. Chem. Sci. 2021, 12, 976–982. [Google Scholar] [CrossRef]
- Kishore, J.; Thiyagarajan, S.; Gunanathan, C. Ruthenium(II)-catalysed direct synthesis of ketazines using secondary alcohols. Chem. Commun. 2019, 55, 4542–4545. [Google Scholar] [CrossRef]
- Gradiski, M.V.; Kharat, A.N.; Ong, M.S.E.; Lough, A.J.; Smith, S.A.M.; Morris, R.H. A one-step preparation of tetradentate ligands with nitrogen and phosphorus donors by reductive amination and representative iron complexes. Inorg. Chem. 2020, 59, 11041–11053. [Google Scholar] [CrossRef] [PubMed]
- Bolzati, C.; Salvarese, N.; Spolaore, B.; Vittadini, A.; Forrer, D.; Brunello, S.; Ghiani, S.; Maiocchi, A. Water-soluble [Tc(N)(PNP)] moiety for room-temperature 99mTc labeling of sensitive target vectors. Mol. Pharm. 2022, 19, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Han, S.; Ke, M.; Ning, Y.; Chen, F.-E. Ligand-enabled palladium-catalyzed hydroesterification of vinyl arenes with high linear selectivity to access 3-arylpropanoate esters. Chem. Commun. 2022, 58, 3921–3924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ning, Y.; Ye, B.; Ru, T.; Chen, F.-E. Water-soluble diphosphine ligands for rhodium-catalyzed branch-selective hydroaminomethylation of vinyl arenes with anilines in water. Green Chem. 2022, 24, 4420–4424. [Google Scholar] [CrossRef]
- Liang, L.-C.; Liao, S.-M.; Zou, X.-R. Facial-meridional isomerization and reductive elimination in [(R2P-o-C6H4)2N]PtMe3 (R = Ph, iPr). Inorg. Chem. 2021, 60, 15118–15123. [Google Scholar] [CrossRef]
- Bacciu, D.; Chen, C.-H.; Surawatanawong, P.; Foxman, B.M.; Ozerov, O.V. High-spin manganese(II) complexes of an amido/bis(phosphine) PNP ligand. Inorg. Chem. 2010, 49, 5328–5334. [Google Scholar] [CrossRef]
- Karasik, A.A.; Balueva, A.S.; Moussina, E.I.; Naumov, R.N.; Dobrynin, A.B.; Krivolapov, D.B.; Litvinov, I.A.; Sinyashin, O.G. 1,3,6-Azadiphosphacycloheptanes: A novel type of heterocyclic diphosphines. Heteroatom Chem. 2008, 19, 125–132. [Google Scholar] [CrossRef]
- Musina, E.I.; Wittmann, T.I.; Strelnik, I.D.; Naumova, O.E.; Karasik, A.A.; Krivolapov, D.B.; Islamov, D.R.; Kataeva, O.N.; Sinyashin, O.G.; Lönnecke, P.; et al. Influence of the rac–meso isomerization of seven-membered cyclic bisphosphines on the predominant formation of chelate complexes. Polyhedron 2015, 100, 344–350. [Google Scholar] [CrossRef]
- Stewart, M.P.; Ho, M.-H.; Wiese, S.; Lindstrom, M.L.; Thogerson, C.E.; Raugei, S.; Bullock, R.M.; Helm, M.L. High catalytic activity for hydrogen production using nickel electrocatalysts with seven-membered cyclic diphosphine ligands containing one pendant amine. J. Am. Chem. Soc. 2013, 135, 6033–6046. [Google Scholar] [CrossRef]
- Hobballah, A.; Lounissi, S.; Motei, R.; Elleouet, C.; Pétillon, F.Y.; Schollhammer, P. Synthesis, characterization and electrochemical reductive properties of complexes [Fe2(CO)4(κ2-PPh2NR2)(μ-dithiolato)] related to the H-cluster of [FeFe]-H2ases. Eur. J. Inorg. Chem. 2021, 205–216. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Heiden, Z.M.; Chambers, G.M.; Johnson, S.I.; Bullock, R.M.; Mock, M.T. Catalytic ammonia oxidation to dinitrogen by hydrogen atom abstraction. Angew Chem. Int. Ed. 2019, 58, 11618–11624. [Google Scholar] [CrossRef] [PubMed]
- Karasik, A.A.; Naumov, R.N.; Sommer, R.; Sinyashin, O.G.; Hey-Hawkins, E. Water-soluble aminomethyl(ferrocenylmethyl)phosphines and their trinuclear transition metal complexes. Polyhedron 2002, 21, 2251–2256. [Google Scholar] [CrossRef]
- Musina, E.I.; Khrizanforova, V.V.; Strelnik, I.D.; Valitov, M.I.; Spiridonova, Y.S.; Krivolapov, D.B.; Litvinov, I.A.; Kadirov, M.K.; Lönnecke, P.; Hey-Hawkins, E.; et al. New functional cyclic aminomethylphosphine ligands for the construction of catalysts for electrochemical hydrogen transformations. Chem. Eur. J. 2014, 20, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Strelnik, I.D.; Dayanova, I.R.; Kolesnikov, I.E.; Fayzullin, R.R.; Litvinov, I.A.; Samigullina, A.I.; Gerasimova, T.P.; Katsyuba, S.A.; Musina, E.I.; Karasik, A.A. The assembly of unique hexanuclear copper(I) complexes with effective white luminescence. Inorg. Chem. 2019, 58, 1048–1057. [Google Scholar] [CrossRef]
- Dayanova, I.R.; Shamsieva, A.V.; Strelnik, I.D.; Gerasimova, T.P.; Kolesnikov, I.E.; Fayzullin, R.R.; Islamov, D.R.; Saifina, A.F.; Musina, E.I.; Hey-Hawkins, E.; et al. Assembly of heterometallic AuICu2I2 cores on the scaffold of NPPN-bridging cyclic bisphosphine. Inorg. Chem. 2021, 60, 5402–5411. [Google Scholar] [CrossRef]
- Dayanova, I.; Khabibullin, R.; Strelnik, I.; Musina, E.; Karasik, A.; Sinyashin, O. Synthesis of palladium(II) complexes of N-p-iodophenyl substituted 1,5-diaza-3,7-diphosphacyclooctanes. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 515–516. [Google Scholar] [CrossRef]
- Wittmann, T.I.; Musina, E.I.; Krivolapov, D.B.; Litvinov, I.A.; Kondrashova, S.A.; Latypov, S.K.; Karasik, A.A.; Sinyashin, O.G. Covalent self-assembly of the specific RSSR isomer of 14-membered tetrakisphosphine. Dalton Trans. 2017, 46, 12417–12420. [Google Scholar] [CrossRef]
- Mock, M.T.; Chen, S.; O’Hagan, M.; Rousseau, R.; Dougherty, W.G.; Kassel, W.S.; Bullock, R.M. Dinitrogen reduction by a chromium(0) complex supported by a 16-membered phosphorus macrocycle. J. Am. Chem. Soc. 2013, 135, 11493–11496. [Google Scholar] [CrossRef]
- Musina, E.I.; Wittmann, T.I.; Musin, L.I.; Balueva, A.S.; Shpagina, A.S.; Litvinov, I.A.; Lönnecke, P.; Hey-Hawkins, E.; Karasik, A.A.; Sinyashin, O.G. Dynamic covalent chemistry approach toward 18-membered P4N2 macrocycles and their nickel(II) complexes. J. Org. Chem. 2020, 85, 14610–14618. [Google Scholar] [CrossRef]
- Tronic, T.A.; Kaminsky, W.; Coggins, M.K.; Mayer, J.M. Synthesis, protonation, and reduction of ruthenium-peroxo complexes with pendent nitrogen bases. Inorg. Chem. 2012, 51, 10916–10928. [Google Scholar] [CrossRef] [PubMed]
- Kireev, N.V.; Kiryutin, A.S.; Pavlov, A.A.; Yurkovskaya, A.V.; Musina, E.I.; Karasik, A.A.; Shubina, E.S.; Ivanov, K.L.; Belkova, N.V. Nickel(II) dihydrogen and hydride complexes as the intermediates of H2 heterolytic splitting by nickel diazadiphosphacyclooctane complexes. Eur. J. Inorg. Chem. 2021, 4265–4272. [Google Scholar] [CrossRef]
- Gunasekara, T.; Tong, Y.; Speelman, A.L.; Erickson, J.D.; Appel, A.M.; Hall, M.B.; Wiedner, E.S. Role of high-spin species and pendant amines in electrocatalytic alcohol oxidation by a nickel phosphine complex. ACS Catal. 2022, 12, 2729–2740. [Google Scholar] [CrossRef]
- Chapple, D.E.; Hoffer, M.A.; Boyle, P.D.; Blacquiere, J.M. Alkyne hydrofunctionalization mechanism including an off-cycle alkoxycarbene deactivation complex. Organometallics 2022, 41, 1532–1542. [Google Scholar] [CrossRef]
- Gross, M.A.; Reynal, A.; Durrant, J.R.; Reisner, E. Versatile photocatalytic systems for H2 generation in water based on an efficient DuBois-type nickel catalyst. J. Am. Chem. Soc. 2014, 136, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Engelhard, M.H.; Lense, S.; Roberts, J.A.S.; Bullock, R.M. Covalent attachment of diphosphine ligands to glassy carbon electrodes via Cu-catalyzed alkyne-azide cycloaddition. Metallation with Ni(II). Dalton Trans. 2015, 44, 12225–12233. [Google Scholar] [CrossRef]
- Rodriguez-Maciá, P.; Dutta, A.; Lubitz, W.; Shaw, W.J.; Rüdiger, O. Direct comparison of the performance of a bio-inspired synthetic nickel catalyst and a [NiFe]-hydrogenase, both covalently attached to electrodes. Angew. Chem. Int. Ed. 2015, 54, 12303–12307. [Google Scholar] [CrossRef]
- Brunner, F.M.; Neville, M.L.; Kubiak, C.P. Investigation of immobilization effects on Ni(P2N2)2 electrocatalysts. Inorg. Chem. 2020, 59, 16872–16881. [Google Scholar] [CrossRef]
- Nakajima, T.; Maeda, M.; Matsui, A.; Nishigaki, M.; Kotani, M.; Tanase, T. Unsymmetric dinuclear RhI2 and RhIRhIII complexes supported by tetraphosphine ligands and their reactivity of oxidative protonation and reductive dichlorination. Inorg. Chem. 2022, 61, 1102–1117. [Google Scholar] [CrossRef]
- van Beek, C.B.; van Leest, N.P.; Lutz, M.; de Vos, S.D.; Klein Gebbink, R.J.M.; de Bruin, B.; Broere, D.L.J. Combining metal–metal cooperativity, metal–ligand cooperativity and chemical non-innocence in diiron carbonyl complexes. Chem. Sci. 2022, 13, 2094–2104. [Google Scholar] [CrossRef]
- Kounalis, E.; Lutz, M.; Broere, D.L.J. Tuning the bonding of a μ-mesityl ligand on dicopper(I) through a proton-responsive expanded PNNP pincer ligand. Organometallics 2020, 39, 585–592. [Google Scholar] [CrossRef]
- Gautam, M.; Tanaka, S.; Sekiguchi, A.; Nakajima, Y. Long-range metal–ligand cooperation by iron hydride complexes bearing a phenanthroline-based tetradentate PNNP ligand. Organometallics 2021, 40, 3697–3702. [Google Scholar] [CrossRef]
- Takeshita, T.; Sato, K.; Nakajima, Y. Selective hydrosiloxane synthesis via dehydrogenative coupling of silanols with hydrosilanes catalysed by Fe complexes bearing a tetradentate PNNP ligand. Dalton Trans. 2018, 47, 17004–17010. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Kamiyama, H.; Okamoto, K.; Irimajiri, M.; Mizutani, T.; Koike, K.; Sekine, A.; Ishitani, O. Highly efficient and robust photocatalytic systems for CO2 reduction consisting of a Cu(I) photosensitizer and Mn(I) catalysts. J. Am. Chem. Soc. 2018, 140, 17241–17254. [Google Scholar] [CrossRef]
- Naina, V.R.; Krätschmer, F.; Roesky, P.W. Selective coordination of coinage metals using orthogonal ligand scaffolds. Chem. Commun. 2022, 58, 5332–5346. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, M.; Kehry, M.; Lebedkin, S.; Kappes, M.M.; Klopper, W.; Roesky, P.W. Bi- and trinuclear coinage metal complexes of a PNNP ligand featuring metallophilic interactions and an unusual charge separation. Dalton Trans. 2021, 50, 13412–13420. [Google Scholar] [CrossRef]
- Dahlen, M.; Seifert, T.P.; Lebedkin, S.; Gamer, M.T.; Kappes, M.M.; Roesky, P.W. Tetra- and hexanuclear string complexes of the coinage metals. Chem. Commun. 2021, 57, 13146–13149. [Google Scholar] [CrossRef]
- Cazorla, C.; Casimiro, L.; Arif, T.; Deo, C.; Goual, N.; Retailleau, P.; Métivier, R.; Xie, J.; Voituriez, A.; Marinetti, A.; et al. Synthesis and properties of photoswitchable diphosphines and gold(I) complexes derived from azobenzenes. Dalton Trans. 2021, 50, 7284–7292. [Google Scholar] [CrossRef]
- Bestgen, S.; Schoo, C.; Neumeier, B.L.; Feuerstein, T.J.; Zovko, C.; Köppe, R.; Feldmann, C.; Roesky, P.W. Intensely photoluminescent diamidophosphines of the alkaline-earth metals, aluminum, and zinc. Angew. Chem. Int. Ed. 2018, 57, 14265–14269. [Google Scholar] [CrossRef]
- Hatzis, G.P.; Thomas, C.M. Metal–ligand cooperativity across two sites of a square planar iron(II) complex ligated by a tetradentate PNNP ligand. Chem. Commun. 2020, 56, 8611–8614. [Google Scholar] [CrossRef]
- Lee, K.; Thomas, C.M. Nickel-templated replacement of phosphine substituents in a tetradentate bis(amido)bis(phosphine) ligand. Inorg. Chem. 2021, 60, 17348–17356. [Google Scholar] [CrossRef] [PubMed]
- Zovko, C.; Bestgen, S.; Schoo, C.; Görner, A.; Goicoechea, J.M.; Roesky, P.W. A phosphine functionalized β-diketimine ligand for the synthesis of manifold metal complexes. Chem. Eur. J. 2020, 26, 13191–13202. [Google Scholar] [CrossRef] [PubMed]
- Marlier, E.E.; Seong, C.M.; Brunclik, S.A.; Nevins, M.H.; Nolan, E.L.; Olson, A.K.; Osnaya, M.; Reuter, A.; Swanson, M.E.; Wood, O.G.H.; et al. Synthesis and structures of a family of hybrid donor N2P2 beta-diketiminate zinc complexes. Polyhedron 2021, 201, 115150. [Google Scholar] [CrossRef]
- Wei, D.; Bruneau-Voisine, A.; Valyaev, D.A.; Lugan, N.; Sortais, J.-B. Manganese catalyzed reductive amination of aldehydes using hydrogen as a reductant. Chem. Commun. 2018, 54, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Lai, Z.; Huang, K.-W. Selective catalytic hydrogenation of arenols by a well-defined complex of ruthenium and phosphorus-nitrogen PN3-pincer ligand containing a phenanthroline backbone. ACS Catal. 2017, 7, 4446–4450. [Google Scholar] [CrossRef]
- Barman, S.; Jaseer, E.A.; Garcia, N.; Elanany, M.; Khawaji, M.; Xu, W.; Lin, S.; Alasiri, H.; Akhtar, M.N.; Theravalappil, R. A rational approach towards selective ethylene oligomerization via PNP-ligand design with an N-triptycene functionality. Chem. Commun. 2022, 58, 10044–10047. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, T.H.; Baek, J.W.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Lee, B.Y. Extremely active ethylene tetramerization catalysts avoiding the use of methylaluminoxane: [iPrN{P(C6H4-p-SiR3)2}2CrCl2]+[B(C6F5)4]−. ChemCatChem 2019, 11, 4351–4359. [Google Scholar] [CrossRef]
- Alam, F.; Fan, H.; Dong, C.; Zhang, J.; Ma, J.; Chen, Y.; Jiang, T. Chromium catalysts stabilized by alkylphosphanyl PNP ligands for selective ethylene tri-/tetramerization. J. Catal. 2021, 404, 163–173. [Google Scholar] [CrossRef]
- Levin, M.D.; Toste, F.D. Gold-catalyzed allylation of aryl boronic acids: Accessing cross-coupling reactivity with gold. Angew. Chem. Int. Ed. 2014, 53, 6211–6215. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, X.-A.; Yan, Z.; Mu, L.; Ma, J.; Zhu, C.; Xie, J. Manganese-catalysed divergent silylation of alkenes. Nat. Chem. 2021, 13, 182–190. [Google Scholar] [CrossRef]
- Kathewad, N.; Anagha, M.C.; Parvin, N.; Parambath, S.; Parameswaran, P.; Khan, S. Facile Buchwald-Hartwig coupling of sterically encumbered substrates effected by PNP ligands. Dalton Trans. 2019, 48, 2730–2734. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Pan, R.-C.; Chen, M.; Liu, Y.; Chen, C.; Lu, X.-B. Synthesis of nonalternating polyketones using cationic diphosphazane monoxide-palladium complexes. J. Am. Chem. Soc. 2021, 143, 10743–10750. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Ren, B.-H.; Li, S.-H.; Song, Y.-H.; Jiao, S.; Zou, C.; Chen, C.; Lu, X.-B.; Liu, Y. Cationic P,O-coordinated nickel(II) catalysts for carbonylative polymerization of ethylene: Unexpected product selectivity via subtle electronic variation. Angew. Chem. Int. Ed. 2022, e202204126. [Google Scholar]
- Wiedner, E.S.; Appel, A.M.; Raugei, S.; Shaw, W.J.; Bullock, R.M. Molecular catalysts with diphosphine ligands containing pendant amines. Chem. Rev. 2022, 122, 12427–12474. [Google Scholar] [CrossRef] [PubMed]
- Norouziyanlakvan, S.; Rao, G.K.; Ovens, J.; Gabidullin, B.; Richeson, D. Electrocatalytic H2 generation from water relying on cooperative ligand electron transfer in “PN3P” pincer-supported NiII complexes. Chem. Eur. J. 2021, 27, 13518–13522. [Google Scholar] [CrossRef]
- Kamada, K.; Jung, J.; Kametani, Y.; Wakabayashi, T.; Shiota, Y.; Yoshizawa, K.; Bae, S.H.; Muraki, M.; Naruto, M.; Sekizawa, K.; et al. Importance of steric bulkiness of iridium photocatalysts with PNNP tetradentate ligands for CO2 reduction. Chem. Commun. 2022, 58, 9218–9221. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, M.B. The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments. Molecules 2022, 27, 6293. https://doi.org/10.3390/molecules27196293
Smith MB. The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments. Molecules. 2022; 27(19):6293. https://doi.org/10.3390/molecules27196293
Chicago/Turabian StyleSmith, Martin B. 2022. "The Backbone of Success of P,N-Hybrid Ligands: Some Recent Developments" Molecules 27, no. 19: 6293. https://doi.org/10.3390/molecules27196293