A Dual Functional Artificial SEI Layer Based on a Facile Surface Chemistry for Stable Lithium Metal Anode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Construction of the Dual Functional Artificial Layer
2.2. Mechanism of the Uniform Li+ Distribution
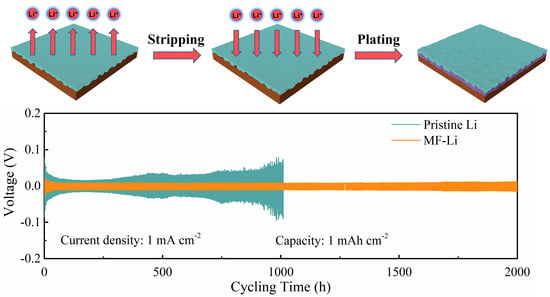
2.3. Electrochemical Evaluation of the Dual Functional Artificial Layer
2.4. Battery Performance
3. Experimental Section
3.1. Materials
3.2. Electrode Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yue, M.; Liu, C.; Zhang, H.; Yu, Y.; Li, X. Long cycle life lithium metal batteries enabled with upright lithium anode. Adv. Funct. Mater. 2019, 29, 1806752. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Shen, Y.; Wu, J.; Zhao, Z.; Zhong, C. Confronting the challenges in lithium anodes for lithium metal batteries. Adv. Sci. 2021, 8, 2101111. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pei, A.; Lin, D.; Xie, J.; Yang, A.; Xu, J. Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode. Adv. Energy Mater. 2019, 9, 1900858. [Google Scholar] [CrossRef]
- Dai, H.; Dong, J.; Wu, M.; Hu, Q.; Wang, D.; Zuin, L.; Chen, N.; Lai, C.; Zhang, G.X.; Sun, S.H. Cobalt-phthalocyanine-derived molecular isolation layer for highly stable lithium anode. Angew. Chem. Int. Ed. 2021, 60, 19852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Li, G.; Zhang, C.J.; Liu, X.J.; Luo, J.Y. Incorporating ionic paths into 3D conducting scaffolds for high volumetric and areal capacity, high rate lithium-metal anodes. Adv. Mater. 2018, 30, 1801328. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jin, H.J.; Yun, Y.S. Advances in the design of 3D-structured electrode materials for lithium-metal anodes. Adv. Mater. 2020, 32, 2002193. [Google Scholar] [CrossRef]
- Jang, E.K.; Ahn, J.; Yoon, S.; Cho, K.Y. High dielectric, robust composite protective layer for dendrite-free and LiPF6 degradation-free lithium metal anode. Adv. Funct. Mater. 2019, 29, 1905078. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, Y.; Ji, S.; Yuan, Z.; Liu, J.; Zhu, J. In situ construction a stable protective layer in polymer electrolyte for ultralong lifespan solid-state lithium metal batteries. Adv. Sci. 2022, 9, 2104277. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, J.; He, J.; Wu, M.; Qi, S.; Wang, H.; Li, F.; Ma, J. Optimizing electrode/electrolyte interphases and Li-ion flux/solvation for lithium-metal batteries with qua-functional heptafluorobutyric anhydride. Angew. Chem. Int. Ed. 2021, 60, 20717. [Google Scholar] [CrossRef]
- Yu, Z.; Mackanic, D.G.; Michaels, W.; Lee, M.; Pei, A.; Feng, D.; Zhang, Q.; Tsao, Y.; Amanchukwu, C.V.; Yan, X.; et al. A dynamic, electrolyte-blocking, and single-ion-conductive network for stable lithium-metal anodes. Joule 2019, 3, 2761–2776. [Google Scholar] [CrossRef]
- Ma, Q.; Yue, J.; Fan, M.; Tan, S.J.; Zhang, J.; Wang, W.P. Formulating the electrolyte towards high-energy and safe rechargeable lithium-metal batteries. Angew. Chem. Int. Ed. 2021, 60, 16554–16560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhao, Y.; Choi, J.W.; Coskun, A. Ionic liquid functionalized gel polymer electrolytes for stable lithium metal batteries. Angew. Chem. Int. Ed. 2021, 133, 22973–22978. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Xin, L. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2017, 2, 17083. [Google Scholar] [CrossRef]
- Li, Z.; Peng, M.; Zhou, X.; Shin, K.; Tunmee, S.; Zhang, X. In situ chemical lithiation transforms diamond-like carbon into an ultrastrong ion conductor for dendrite-free lithium-metal anodes. Adv. Mater. 2021, 33, 2100793. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yao, Y.X.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Huang, J.Q. Lithium nitrate solvation chemistry in carbonate electrolyte sustains high-voltage lithium metal batteries. Angew. Chem. Int. Ed. 2018, 57, 14055. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, A.C.; Shetty, P.P.; Kondekar, N. Efficient low-temperature cycling of lithium metal anodes by tailoring the solid-electrolyte interphase. ACS Energy Lett. 2020, 5, 2411–2420. [Google Scholar] [CrossRef]
- Fu, J.; Ji, X.; Chen, J.; Chen, L.; Fan, X.; Mu, D. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. 2020, 59, 22194. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.P.; Yao, N.; Xie, J.; Shi, P.; Sun, S.Y.; Jin, C.B. Modification of nitrate ion enables stable solid electrolyte interphase in lithium metal batteries. Angew. Chem. Int. Ed. 2022, 61, e202201406. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial soft–rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 2018, 28, 1705838. [Google Scholar] [CrossRef]
- Jiang, G.; Li, K.; Yu, F.; Li, X.L.; Mao, J.Y.; Jiang, W.W.; Sun, F.G.; Dai, B.; Li, Y.S. Robust artificial solid-electrolyte interfaces with biomimetic ionic channels for dendrite-free li metal anodes. Adv. Energy Mater. 2021, 11, 2003496. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Chen, G.; Bai, Y.; Wu, C. Porous LiF layer fabricated by a facile chemical method toward dendrite-free lithium metal anode. J. Energy Chem. 2019, 37, 197–203. [Google Scholar] [CrossRef]
- Lang, S.Y.; Shen, Z.Z.; Hu, X.C.; Shi, Y.; Guo, Y.G.; Wan, L.J. Tunable structure and dynamics of solid electrolyte interphase at lithium metal anode. Nano Energy 2020, 75, 104967. [Google Scholar] [CrossRef]
- Jin, D.; Roh, Y.; Jo, T.; Ryou, M.H.; Lee, H.Y.; Lee, Y.M. Robust cycling of ultrathin Li metal enabled by nitrate-preplanted Li powder composite. Adv. Energy Mater. 2021, 11, 2003769. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Lai, C. Li-containing alloys beneficial for stabilizing lithium anode: A review. Eng. Rep. 2021, 3, 12339. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, F.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y. Highly stable lithium-sulfur batteries achieved by a SnS/porous carbon nanosheet architecture modified celgard separator. Adv. Funct. Mater. 2020, 30, 2006297. [Google Scholar] [CrossRef]
- Wang, L.; Fu, S.; Zhao, T.; Qian, J.; Chen, N.; Li, L.; Wu, F.; Chen, R.J. In situ formation of a LiF and Li-Al alloy anode protected layer on a Li metal anode with enhanced cycle life. J. Mater. Chem. A 2020, 8, 1247–1253. [Google Scholar] [CrossRef]
- Liang, X.; Pang, Q.; Kochetkov, I.R.; Sempere, M.S.; Huang, H.; Sun, X.Q.; Nazar, L.F. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2017, 2, 17119. [Google Scholar] [CrossRef]
- Song, C.L.; Li, Z.H.; Ma, L.Y.; Li, M.Z.; Huang, S.; Hong, X.J.; Cai, Y.P.; Lan, Y.Q. Single-atom zinc and anionic framework as janus separator coatings for efficient inhibition of lithium dendrites and shuttle effect. ACS Nano 2021, 15, 13436–13443. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Gao, L.; Lu, Y.; Archer, L.A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 2017, 5, 3483–3492. [Google Scholar] [CrossRef]
- Ouyang, Y.; Guo, Y.; Li, D.; Wei, Y.; Zhai, T.; Li, H. Single additive with dual functional-ions for stabilizing lithium anodes. ACS Appl. Mater. Interfaces 2019, 11, 11360–11368. [Google Scholar] [CrossRef]
- Zhang, H.; Ju, S.; Xia, G.; Yu, X.B. Identifying the positive role of lithium hydride in stabilizing Li metal anodes. Sci. Adv. 2022, 8, 18245. [Google Scholar] [CrossRef]
- Jagannathan, M.; Chandran, K.S.R. Electrochemical charge/discharge behavior and phase transitions during cell cycling of Li(Mg) alloy anodes for high capacity li ion batteries. J. Electrochem. Soc. 2013, 160, 1922–1926. [Google Scholar] [CrossRef]
- Gao, P.; Wu, H.; Zhang, X.; Jia, H.; Kim, J.M.; Zhang, J.G.; Xu, W. Optimization of magnesium-doped lithium metal anode for high performance lithium metal batteries through modeling and experiment. Angew. Chem. Int. Ed. 2021, 133, 16642–16649. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Naik, D.; Gile., J.L. Electrochemical properties of Li-Mg alloy electrodes for lithium batteries. J. Power Sources 2001, 92, 70. [Google Scholar] [CrossRef]
- Hu, A.; Chen, W.; Du, X.; Hu, Y.; Lei, T.; Wang, H.; Xue, L.; Li, Y.; Sun, H.; Yan, Y.; et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energy Environ. Sci. 2021, 14, 4115–4124. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blochl, P.E. Projected augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, H.; Meyerson, M.L.; Rodriguez, R.; Kawashima, K.; Weeks, J.A.; Sun, H.; Xie, Q.; Lin, J.; Henkelman, G.; et al. Li-Zn overlayer to facilitate uniform lithium deposition for lithium metal batteries. ACS Appl. Mater. Interfaces 2021, 13, 9985–9993. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhang, R.; Fu, C.; Xiao, R.; Li, R.; Ma, Y.; Wang, J.; Gao, Y.; Yin, G.; Zuo, P. Stable lithium anode enabled by biphasic hybrid SEI layer toward high-performance lithium metal batteries. Chem. Eng. J. 2022, 433, 133570. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Liu, M.; Wang, C.; Zhong, Y.; Wang, H.; Cheng, X.; Liu, S.; Cao, G. Facile and scalable engineering of a heterogeneous microstructure for uniform, stable and fast lithium plating/stripping. J. Mater. Chem. A 2019, 7, 19104–19111. [Google Scholar] [CrossRef]
- Li, S.; Fan, L.; Lu, Y. Rational design of robust-flexible protective layer for safe lithium metal battery. Energy Storage Mater. 2019, 18, 205–212. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, L.; Gu, Y.; Zhao, L.; Jing, Y.; Mu, Q.; Su, Y.; Yuan, X.; Peng, Y.; Deng, Z. Insulative ion-conducting lithium selenide as the artificial solid-electrolyte interface enabling heavy-duty lithium metal operations. Nano Lett. 2021, 21, 7354–7362. [Google Scholar] [CrossRef]
- Wang, H.; Wu, L.; Xue, B.; Wang, F.; Luo, Z.; Zhang, X.; Calvez, L.; Fan, P.; Fan, B. Interfaces, improving cycling stability of the lithium anode by a spin-coated high-purity Li3PS4 artificial SEI layer. ACS Appl. Mater. Interfaces 2022, 14, 15214–15224. [Google Scholar] [CrossRef]
- Shen, X.; Li, Y.; Qian, T.; Liu, J.; Zhou, J.; Yan, C.; Goodenough, J. Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 2019, 10, 900. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wu, F.; Chen, N.; Yang, T.; Liang, Y.; Sun, Z.; Luo, G.; Du, J.; Shang, Y.; Feng, M.; et al. A Dual Functional Artificial SEI Layer Based on a Facile Surface Chemistry for Stable Lithium Metal Anode. Molecules 2022, 27, 5199. https://doi.org/10.3390/molecules27165199
Ma Y, Wu F, Chen N, Yang T, Liang Y, Sun Z, Luo G, Du J, Shang Y, Feng M, et al. A Dual Functional Artificial SEI Layer Based on a Facile Surface Chemistry for Stable Lithium Metal Anode. Molecules. 2022; 27(16):5199. https://doi.org/10.3390/molecules27165199
Chicago/Turabian StyleMa, Yue, Feng Wu, Nan Chen, Tianyu Yang, Yaohui Liang, Zhaoyang Sun, Guangqiu Luo, Jianguo Du, Yanxin Shang, Mai Feng, and et al. 2022. "A Dual Functional Artificial SEI Layer Based on a Facile Surface Chemistry for Stable Lithium Metal Anode" Molecules 27, no. 16: 5199. https://doi.org/10.3390/molecules27165199