Effects of Different Denaturants on the Properties of a Hot-Pressed Peanut Meal-Based Adhesive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
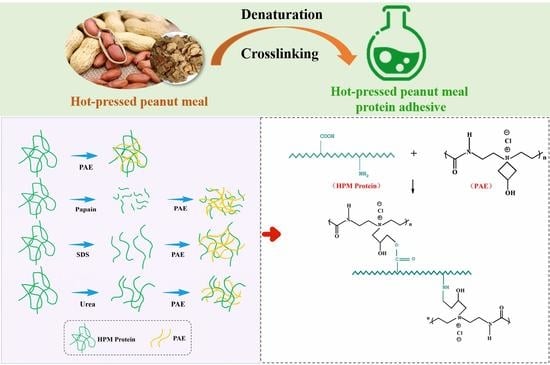
2.2. Preparation of HPM-Based Adhesive
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Three-Ply Plywood Preparation and Bonding Strength Test
2.5. Thermogravimetry (TGA)
2.6. Mass Loss (ML) and Moisture Uptake Value (MUV) Test
2.7. Viscosity Test
2.8. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. FTIR Analysis
3.2. Bonding Strength Measurement
3.3. TGA Analysis
3.4. ML and MUV Measurement
3.5. Viscosity Measurement
3.6. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, W.; Li, F.; Liu, X.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Borate chemistry inspired by cell walls converts soy protein into high-strength, antibacterial, flame-retardant adhesive. Green Chem. 2020, 22, 1319–1928. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Bai, Y.; Gao, Z. Economical improvement on the performances of a soybean flour-based adhesive for wood composites via montmorillonite hybridization. Compos. B. Eng. 2021, 217, 108920. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Wang, Y.; Liao, Y.; Xiong, H.; Selomulya, C.; Hu, J.; Zhao, Q. Complete waste recycling strategies for improving the accessibility of rice protein films. Gree Chem. 2020, 22, 490–503. [Google Scholar] [CrossRef]
- Ai, X.; Feng, S.; Shui, T.; Kakkar, H.; Xu, C.C. Effects of alcell lignin methylolation and lignin adding stage on lignin-based phenolic adhesives. Molecules 2021, 26, 6762. [Google Scholar] [CrossRef] [PubMed]
- Watcharakitti, J.; Win, E.E.; Nimnuan, J.; Smith, S.M. Modified starch-based adhesives: A review. Polymers 2022, 14, 2023. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.; Lin, X.; Chen, H.; Gao, Q.; Li, J. High-performance adhesives formulated from soy protein isolate and bio-based material hybrid for plywood production. J. Clean. Prod. 2022, 353, 131587. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, A.; Wang, Q. Peanut meal as plywood adhesives: Preparation and characterization. J. Adhes. Sci. Technol. 2018, 32, 2450–2463. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, Q.; Li, T.; Zhang, S.; Wang, B.; Yue, H.; Liu, H.; Yang, J.; Wang, Q. Preparation and characterization of birch plywood prepared by hot-pressed peanut meal adhesive. Int. J. Adhes. Adhes. 2022, 117, 103186. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Liu, B.; Du, Y.; Liu, K. Peanut meal-based wood adhesives enhanced by urea and epichlorohydrin. R. Soc. Open Sci. 2019, 6, 191154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, M.; Cao, J.; Li, J.; Li, C. Development of soybeans starch based tough, water resistant and mildew-proof adhesives through multiple cross linking cooperation strategy. J. Clean. Prod. 2021, 321, 129001. [Google Scholar] [CrossRef]
- Liu, H.; Bean, S.; Sun, X.S. Camelina protein adhesives enhanced by polyelectrolyte interaction for plywood applications. Ind. Crops Prod. 2018, 124, 343–352. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Li, X.; Li, J.; Shi, S.Q.; Li, J. Bioinspired interface engineering of soybean meal-based adhesive incorporated with biomineralized cellulose nanofibrils and a functional aminoclay. Chem. Eng. J. 2021, 421, 129820. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Xu, Y.; Li, J.; Shi, S.Q.; Li, J.; Gao, Q. High performance and multifunctional protein-based adhesive produced via phenol-amine chemistry and mineral reinforcement strategy inspired by arthropod cuticles. Chem. Eng. J. 2021, 426, 130852. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Mussel byssus-inspired engineering of synergistic nanointerfacial interactions as sacrificial bonds into carbon nanotube-reinforced soy protein/nanofibrillated cellulose nanocomposites: Versatile mechanical enhancement. Appl. Surf. Sci. 2018, 434, 1086–1100. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Han, Y.; Chen, M.; Zhang, W.; Gao, Q.; Li, J. The effect of enzymolysis on performance of soy protein-based adhesive. Molecules 2018, 23, 2752. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Yao, J.; Shao, Z.; Chen, X. Water-resistant zein-based adhesives. ACS Sustain. Chem. Eng. 2020, 8, 7668–7679. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Li, Y.; Ma, Y. Properties of a new renewable sesame protein adhesive modified by urea in the absence and presence of zinc oxide. RSC Adv. 2017, 7, 46388–46394. [Google Scholar] [CrossRef] [Green Version]
- China National Standard GB/T 9846; Plywood for General Use. Standardization Administration of the Peoples Republic of China: Beijing, China, 2015.
- Xu, Y.; Han, Y.; Li, Y.; Li, J.; Li, J.; Gao, Q. Preparation of a strong, mildew-resistant, and flame-retardant biomimetic multifunctional soy protein adhesive via the construction of an organic-inorganic hybrid multiple-bonding structure. Chem. Eng. J. 2022, 437, 135437. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, Q.; Li, T.; Zhang, Y.; Gao, Q.; Liu, H.; Wang, Q. A novel environmentally friendly hot-pressed peanut meal protein adhesive. J. Clean. Prod. 2021, 327, 129473. [Google Scholar] [CrossRef]
- Yue, L.; Meng, Z.; Yi, Z.; Gao, Q.; Mao, A.; Li, J. Effects of different denaturants on properties and performance of soy protein-based adhesive. Polymers 2019, 11, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Wang, Z.; Pang, H.; Li, Z.; Zhang, W.; Zhang, S.; Li, J.; Li, L. Designing biomimetic microphase-separated motifs to construct mechanically robust plant protein resin with improved water-resistant performance. Macromol. Mater. Eng. 2020, 305, 1900462. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Chen, M.; Luo, J.; Shi, S.Q.; Li, J.; Gao, Q. Constructing a triple network structure to prepare strong, tough, and mildew resistant soy protein adhesive. Compos. B. Eng. 2021, 211, 108677. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Gao, Q.; Zhang, W.; Chen, H.; Li, J.; Shi, S.Q. Multiple crosslinking strategy to achieve high bonding strength and antibacterial properties of double-network soy adhesive. J. Clean. Prod. 2020, 254, 120143. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, Y.; Shi, S.Q.; Gao, Q. Improving bond performance and reducing cross-linker dosage for soy flour adhesives inspired by spider silk. ACS Sustain. Chem. Eng. 2020, 9, 168–179. [Google Scholar] [CrossRef]
- Zeng, G.; Zhou, Y.; Liang, Y.; Zhang, F.; Luo, J.; Li, J.; Fang, Z. A hair fiber inspired bio-based adhesive with high bonding strength and mildew tolerance. Chem. Eng. J. 2022, 434, 134632. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, Z.; Zhang, S.; Li, J. Biomimetic water-in-oil water/pMDI emulsion as an excellent ecofriendly adhesive for bonding wood-based composites. J. Hazard. Mater. 2020, 396, 122722. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Y.; Gao, Q.; Li, J.; Yan, N. From wastes to functions: A new soybean meal and bark-based adhesive. ACS Sustain. Chem. Eng. 2020, 8, 10767–10773. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Li, Y.; Shi, S.Q.; Li, J.; Gao, Q.; Guo, H. Soybean meal-based wood adhesive enhanced by phenol hydroxymethylated tannin oligomer for exterior use. Polymers 2020, 12, 758. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhou, Y.; Zhang, Y.; Gao, Q.; Li, J. An eco-effective soybean meal-based adhesive enhanced with diglycidyl resorcinol ether. Polymers 2020, 12, 954. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Shi, S.Q.; Gao, Q.; Li, J. Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. J. Clean. Prod. 2020, 255, 120303. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wang, Y.; Chang, Z.; Xia, C.; Huang, Z. Soy meal adhesive with high strength and water resistance via carboxymethylated wood fiber-induced crosslinking. Cellulose 2021, 28, 3569–3584. [Google Scholar] [CrossRef]







| Adhesive Sample | HPM (g) | Distilled Water (g) | Denaturant (g) | PAE (g) |
|---|---|---|---|---|
| Pure HPM | 20 | 55 | 0 | 0 |
| HPM-Papain | 20 | 55 | 0.8 | 0 |
| HPM-SDS | 20 | 55 | 0.8 | 0 |
| HPM-Urea | 20 | 55 | 0.8 | 0 |
| HPM-PAE | 20 | 55 | 0 | 16 |
| HPM-Papain-PAE | 20 | 55 | 0.8 | 16 |
| HPM-SDS-PAE | 20 | 55 | 0.8 | 16 |
| HPM-Urea-PAE | 20 | 55 | 0.8 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Guo, Q.; Li, T.; Liu, H.; Wang, Q. Effects of Different Denaturants on the Properties of a Hot-Pressed Peanut Meal-Based Adhesive. Molecules 2022, 27, 4878. https://doi.org/10.3390/molecules27154878
Qu Y, Guo Q, Li T, Liu H, Wang Q. Effects of Different Denaturants on the Properties of a Hot-Pressed Peanut Meal-Based Adhesive. Molecules. 2022; 27(15):4878. https://doi.org/10.3390/molecules27154878
Chicago/Turabian StyleQu, Yang, Qin Guo, Tian Li, Hongzhi Liu, and Qiang Wang. 2022. "Effects of Different Denaturants on the Properties of a Hot-Pressed Peanut Meal-Based Adhesive" Molecules 27, no. 15: 4878. https://doi.org/10.3390/molecules27154878