pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Beads’ Content
2.1.1. Carrageenan/Alginate Beads
2.1.2. Carrageenan/Alginate-Curcumin Beads
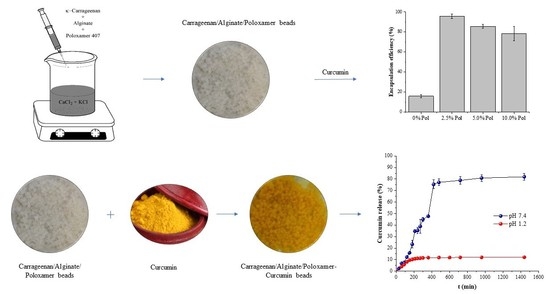
2.1.3. Carrageenan/Alginate/Poloxamer and Carrageenan/Alginate/Poloxamer-Curcumin Beads
2.2. Characterization of Beads by FTIR
2.3. SEM Analysis
2.4. Thermal Characteristics
2.4.1. Thermogravimetric Study
2.4.2. Differential Scanning Calorimetry
2.5. In Vitro Release of Curcumin
2.6. The Kinetics of Release
2.7. Inflammation Study
2.8. In Vivo Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Carrageenan/Alginate Beads (Car/Alg)
3.3. Preparation of Carrageenan/Alginate/Poloxamer Beads (Car/Alg/Pol)
3.4. Preparation of Curcumin-Incorporated Beads (Car/Alg-Cur and Car/Alg/Pol-Cur)
3.5. Characterization of the Beads by FTIR and SEM
3.6. Thermal Characterization of Beads
3.7. Degree of Swelling
3.8. Determination of Encapsulation Efficiency
3.9. In Vitro Release of Curcumin
3.10. The Kinetics of Release
3.11. Inflammation Study
3.12. In Vivo Absorption Studies and CUR Plasma Concentration Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Stanić, Z. Curcumin, a compound from natural sources, a true scientific challenge—A review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Ziora, Z.M.; Tamer, T.M.; Khalifa, R.E.; Hassan, M.A.; Mohy-Eldin, M.S.; Blaskovich, M.A.T. Formulation of quaternized aminated chitosan nanoparticles for efficient encapsulation and slow release of curcumin. Molecules 2021, 26, 449. [Google Scholar] [CrossRef] [PubMed]
- Liua, Y.; Liua, D.; Zhua, L.; Gana, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, S.; Kong, D.; Gao, X.; Zhao, Y.; Wang, Z. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharmacol. Res. 2012, 29, 3512–3525. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Dey, S.; Sreenivasan, K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydr. Polym. 2013, 99, 499–507. [Google Scholar] [CrossRef]
- Suna, J.; Bia, C.; Chana, H.M.; Sunc, S.; Zhanga, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro anti-tumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-based complex particles’ protective role on the stability and bioactivity of immobilized curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Hoare, R.T.; Kohane, S.D. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Soppimath, S.K.; Aminabhavi, M.T.; Kulkarni, R.A.; Rudzinski, E.W. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Nerurkar, J.; Jun, H.W.; Price, J.C.; Park, M.O. Controlled-release matrix tablets of ibuprofen using cellulose ethers and carrageenans: Effect of formulation factors on dissolution rates. Eur. J. Pharm. Biopharm. 2005, 61, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Piyakulawat, P.; Praphairaksit, N.; Chantarasiri, N.; Muangsin, N. Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS PharmSciTech 2007, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Yin, S.; Ma, Z. Ca2+-Triggered pH-response sodium alginate hydrogel precipitation for amplified sandwich-type impedimetric immunosensor of tumor marker. ACS Sens. 2019, 4, 450–455. [Google Scholar]
- Ahmad, M.; Rai, S.M.; Mahmood, A. Hydrogel microparticles as an emerging tool in pharmaceutical field: A review. Adv. Polym. Technol. 2016, 35, 121–128. [Google Scholar] [CrossRef]
- Dutta, S.; Samanta, P.; Dhara, D. Temperature, pH and redox responsive cellulose based hydrogels for protein delivery. Int. J. Biol. Macromol. 2016, 87, 92–100. [Google Scholar] [CrossRef]
- Banerjee, S.; Siddiqui, L.; Bhattacharya, S.S.; Kaity, S.; Ghosh, A.; Chattopadhyay, P.; Pandey, A.; Singh, L. Interpenetrating polymer network (IPN) hydrogel microspheres for oral controlled release application. Int. J. Biol. Macromol. 2012, 50, 198–206. [Google Scholar] [CrossRef]
- Lim, H.-P.; Ooi, C.-W.; Tey, B.-T.; Chan, E.S. Controlled delivery of oral insulin aspart using pH-responsive alginate/κ-carrageenan composite hydrogel beads. React. Funct. Polym. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. pH-Sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J. Bioact. Compat. Polym. 2007, 22, 342–356. [Google Scholar] [CrossRef]
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, P.K.; Nair, V.S.; Tamura, H.; Jayakumar, R. Efficient water-soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sowasod, N.; Tanthapanichakoon, W.; Charinpanitkul, T. Hydrogel based oil encapsulation for controlled release of curcumin by using a ternary system of chitosan, kappa-carrageenan, and carboxymethylcellulose sodium salt. LWT–Food Sci. Technol. 2013, 54, 600–605. [Google Scholar] [CrossRef]
- Sarika, P.R.; James, N.R. Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohydr. Polym. 2016, 148, 354–361. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaume, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Paşcalãu, V.; Popescu, V.; Popescu, G.L.; Dudescu, M.C.; Borodi, G.; Dinescu, A.; Perhaiţa, I.; Paul, M. The alginate/k-carrageenan ratio’s influence on the properties of the cross-linked composite films. J. Alloys Compd. 2012, 536S, S418–S423. [Google Scholar] [CrossRef]
- Yan, M.; Chen, T.; Zhang, S.; Lu, T.; Sun, X. A core-shell structured alginate hydrogel beads with tunable thickness of carboxymethyl cellulose coating for pH responsive drug delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Wang, X.; Wang, Q. Development of carboxymethyl chitosan hydrogel beads in alcohol-aqueous binary solvent for nutrient delivery applications. Food Hydrocoll. 2013, 31, 332–339. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Chun, M.K.; Kwak, B.T.; Choi, H.K. Preparation of buccal patch composed of carbopol, poloxamer and hydroxypropyl methylcellulose. Arch. Pharm. Res. 2003, 26, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Yoo, M.-K.; Kim, B.-C.; Kim, S.K.; Lee, H.C.; Cho, C.S. Preparation of semi-interpenetrating polymer networks composed of chitosan and poloxamer. Int. J. Biol. Macromol. 2006, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.G.; Rani, V.V.D.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Bozoglan, B.K.; Polat, T.G. Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-k-carrageenan-Fe3O4 nanocomposite. J. Alloys Compd. 2016, 687, 370–383. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Lang, M. Micelles/sodium-alginate composite gel beads: A new matrix for oral drug delivery of indomethacin. Carbohydr. Polym. 2012, 87, 790–798. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Rizwan, M.; Azeem, M.K.; Mehmood, A.; Khan, K.U.; Qureshi, A.R.; Mahmood, H.A. Kinetics and controlled release of lidocaine from novel carrageenan and alginate-based blend hydrogels. Int. J. Biol. Macromol. 2020, 147, 67–78. [Google Scholar] [CrossRef]
- Sun, X.; Liua, C.; Omer, A.M.; Yang, L.Y.; Ouyang, X.k. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Liao, S.; Huang, Y.; Li, Y.; He, Y.; Tong, Z.; Li, B. Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem. 2014, 155, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Estaca, J.; Balaguer, M.P.; Gavara, R.; Hernandez-Munoz, P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Yen, F.L.; Wu, T.F.; Tzeng, C.W.; Lin, L.T.; Ling, C.C. Curcumin nanoparticles improve the physicochemical properties of curcumin and effectively enhances its antioxidant and antihepatoma activities. J. Agric. Food Chem. 2010, 58, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Reisi-Vanani, A.; Barati, M. Polyvinyl alcohol-sodium alginate blend, composited with 3D-graphene oxide as a controlled release system for curcumin. J. Drug Deliv. Sci. Technol. 2019, 50, 380–387. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Amjad, J.M. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, 01466. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dul, M.; Paluch, K.J.; Kelly, H.; Healy, A.M.; Sasse, A.; Tajber, L. Self-assembled carra-geenan/protamine polyelectrolyte nanoplexes—Investigation of critical parameters governing their formation and characteristics. Carbohydr. Polym. 2015, 123, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ye, L.; Cui, M.; Yang, B.; Li, J.; Sun, H.; Yao, F. Physically crosslinked poly(vinyl alcohol)–carrageenan composite hydrogels: Pore structure stability and cell adhesive ability. RSC Adv. 2015, 5, 78180–78191. [Google Scholar] [CrossRef]
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring. Acta Biomater. 2020, 109, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef] [PubMed]











| Type of Beads | Diameter (mm) | Mass of Curcumin (mg/g of Beads) |
|---|---|---|
| Car/Alg | 1.10 ± 0.06 | / |
| Car/Alg-Cur | 1.03 ± 0.11 | 10.16 ± 0.15 |
| Car/Alg/Pol | 1.23 ± 0.10 | / |
| Car/Alg/Pol-Cur | 1.13 ± 0.10 | 61.21 ± 1.37 |
| pH Value | Zero-Order Kinetics | First-Order Kinetics | Highuchi Model | ||||
|---|---|---|---|---|---|---|---|
| k0 | R2 | kI | R2 | kH | R2 | ||
| 1.2 | 0.0186 | 0.3362 | 0.0261 | 0.2715 | 0.1325 | 0.5068 | |
| 7.4 | 0.0484 | 0.7353 | 0.1205 | 0.5138 | 0.2955 | 0.8815 | |
| Hixon–Crowell model | Baker–Lonsdale model | Korsmeyer–Peppas model | |||||
| kHC | R2 | kBL | R2 | kKP | n | R2 | |
| 1.2 | 0.0319 | 0.7224 | 0.0186 | 0.6286 | 0.4880 | 1.0023 | 0.9968 |
| 7.4 | 0.0473 | 0.9358 | 0.0265 | 0.8893 | 0.1455 | 1.1451 | 0.9620 |
| Formulation | Tmax (h) | Cmax (ng/mL) | AUC (μg·h/mL) |
|---|---|---|---|
| Curcumin | 2 | 89.9 ± 4.1 | 1.04 |
| Car/Alg/Pol-Cur | 2 | 219.7 ± 2.9 | 2.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Miletić Kovačević, M.; Gazdić Janković, M.; Stanić, Z.D. pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. https://doi.org/10.3390/molecules27134045
Postolović KS, Antonijević MD, Ljujić B, Miletić Kovačević M, Gazdić Janković M, Stanić ZD. pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules. 2022; 27(13):4045. https://doi.org/10.3390/molecules27134045
Chicago/Turabian StylePostolović, Katarina S., Milan D. Antonijević, Biljana Ljujić, Marina Miletić Kovačević, Marina Gazdić Janković, and Zorka D. Stanić. 2022. "pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency" Molecules 27, no. 13: 4045. https://doi.org/10.3390/molecules27134045