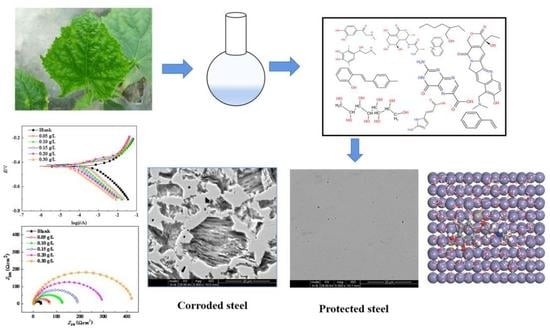
Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis
Abstract
:1. Introduction
2. Results
2.1. Potentiodynamic Polarization Curves
2.2. Electrochemical Impedance Spectroscopy (EIS) Measurements
2.3. Scanning Electron Microscopy (SEM) Observations
2.4. Adsorption Isotherm
2.5. Gas Chromatography and Mass Spectroscopy (GC–MS) Analysis
2.6. Quantum Chemical Calculations
2.7. Molecular Dynamics Simulations (MDS)
2.8. Corrosion Inhibition Mechanism
3. Materials and Methods
3.1. Materials
3.2. ECSL Preparation
3.3. Chemical Composition Analysis
3.4. Electrochemical Techniques
3.5. Surface Morphological Observation
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the corrosion of X70 and Q235 steel in CO2-saturated brine by imidazoline-based inhibitor. J. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Erramli, H.; Cherkaoui, O.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Adsorption and anticorrosive behavior of aromatic epoxy monomers on carbon steel corrosion in acidic solution: Computational studies and sustained experimental studies. RSC Adv. 2019, 9, 14782–14796. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Saha, S.K.; Banerjee, P. Triazole-modified chitosan: A biomacromolecule as a new environmentally benign corrosion inhibitor for carbon steel in a hydrochloric acid solution. RSC Adv. 2019, 9, 14990–15003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, F.; Zhang, W. 4-(Pyridin-4-yl)thiazol-2-amine as an efficient non-toxic inhibitor for mild steel in hydrochloric acid solutions. RSC Adv. 2019, 9, 10454–10464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, C.; Shen, L.; Hao, L.; Mu, X.; Dong, J.; Ke, W.; Liu, J.; Liu, B. Corrosion kinetics and patina evolution of galvanized steel in a simulated coastal-industrial atmosphere. J. Mater. Res. Technol. 2019, 35, 2345–2356. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.; Yu, L.; Zhao, H.; Sun, C.; Yin, F.; Ke, W. Stress corrosion of pipeline steel under disbonded coating in a SRB-containing environment. Corros. Sci. 2019, 157, 518–530. [Google Scholar] [CrossRef]
- Al-Shihry, S.S.; Sayed, A.R.; Abd El-lateef, H.M. Design and assessment of a novel poly(urethane-semicarbazides) containing thiadiazoles on the backbone of the polymers as inhibitors for steel pipelines corrosion in CO2-saturated oilfield water. J. Mol. Struct. 2020, 1201, 127223. [Google Scholar] [CrossRef]
- Kong, P.; Feng, H.; Chen, N.; Lu, Y.; Li, S.; Wang, P. Polyaniline/chitosan as a corrosion inhibitor for mild steel in acidic medium. RSC Adv. 2019, 9, 9211–9217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Basiony, N.M.; Elgendy, A.; Nady, H.; Migahed, M.A.; Zaki, E.G. Adsorption characteristics and inhibition effect of two Schiff base compounds on corrosion of mild steel in 0.5 M HCl solution: Experimental, DFT studies, and Monte Carlo simulation. RSC Adv. 2019, 9, 10473–10485. [Google Scholar] [CrossRef] [Green Version]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.I.; Mahrous, Y.S. Corrosion inhibition of C-steel in acidic media from fruiting bodies of Melia azedarach L. extract and a synergistic Ni2+ additive. RSC Adv. 2017, 7, 23687–23698. [Google Scholar] [CrossRef] [Green Version]
- Boudjellal, F.; Ouici, H.B.; Guendouzi, A.; Benali, O.; Sehmi, A. Experimental and theoretical approach to the corrosion inhibition of mild steel in acid medium by a newly synthesized pyrazole carbothioamide heterocycle. J. Mol. Struct. 2020, 1199, 127051. [Google Scholar] [CrossRef]
- Hermoso-Diaz, I.A.; Foroozan, A.E.; Flores-De los Rios, J.P.; Landeros-Martinez, L.L.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G. Electrochemical and quantum chemical assessment of linoleic acid as a corrosion inhibitor for carbon steel in sulfuric acid solution. J. Mol. Struct. 2019, 1197, 535–546. [Google Scholar] [CrossRef]
- Abdallah, Y.M.; Shalabi, K.; Bayoumy, N.M. Eco-friendly synthesis, biological activity and evaluation of some new pyridopyrimidinone derivatives as corrosion inhibitors for API 5L X52 carbon steel in 5% sulfamic acid medium. J. Mol. Struct. 2018, 1171, 658–671. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Tantawy, A.H.; Soliman, K.A.; Abd El-Lateef, H.M. Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: A combined empirical and theoretical approaches. J. Mol. Struct. 2020, 1203, 127442. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Tecuapa-Flores, E.D.; Turcio-Ortega, D.; Hernandez, J.G.; Huerta-Aguilar, C.A.; Thangarasu, P. The role of keto group in cyclic ligand 1,4,8,11-tetraazacyclotetradecane-5,7-dione as strong corrosion inhibitor for carbon steel surface: Experimental and theoretical studies. J. Mol. Struct. 2019, 1189, 131–145. [Google Scholar] [CrossRef]
- Anupama, K.K.; Ramya, K.; Joseph, A. Electrochemical and computational aspects of surface interaction and corrosion inhibition of mild steel in hydrochloric acid by Phyllanthus amarus leaf extract (PAE). J. Mol. Liq. 2016, 216, 146–155. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Cui, X.; Chen, D.; Li, G. Experimental and theoretical investigation on corrosion inhibitive properties of steel rebar by a newly designed environmentally friendly inhibitor formula. RSC Adv. 2018, 8, 6507–6518. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Kaya, S. Theoretically and experimentally exploring the corrosion inhibition of N80 steel by pyrazol derivatives in simulated acidizing environment. J. Mol. Struct. 2020, 1206, 127685. [Google Scholar] [CrossRef]
- Nathiya, R.S.; Raj, V. Evaluation of Dryopteris cochleata leaf extracts as green inhibitor for corrosion of aluminium in 1 M H2SO4. Egypt. J. Pet. 2017, 26, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Mehdipour, M.; Ramezanzadeh, B.; Arman, S.Y. Electrochemical noise investigation of Aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H2SO4. J. Ind. Eng. Chem. 2015, 21, 318–327. [Google Scholar] [CrossRef]
- Xhanari, K.; Finšgar, M.; Knez Hrnčič, M.; Maver, U.; Knez, Ž.; Seiti, B. Green corrosion inhibitors for aluminium and its alloys: A review. RSC Adv. 2017, 7, 27299–27330. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.J.; Valdelamar, M.P.; Vazquez, A.E.; Del Valle Perez, P.; Mata, R.; Miralrio, A.; Castro, M. Mycophenolic acid as a corrosion inhibitor of carbon steel in 3% wt. NaCl solution. An experimental and theoretical study. J. Mol. Struct. 2019, 1183, 168–181. [Google Scholar] [CrossRef]
- Pitchaipillai, M.; Raj, K.; Balasubramanian, J.; Periakaruppan, P. Benevolent behavior of Kleinia grandiflora leaf extract as a green corrosion inhibitor for mild steel in sulfuric acid solution. Int. J. Miner. Metall. Mater. 2014, 21, 1083–1095. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Xie, X. Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corros. Sci. 2014, 78, 29–42. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Li, N.; Xie, X. Inhibition effect of bamboo leaves extract on cold rolled steel in Cl3CCOOH solution. J. Mater. Res. Technol. 2017, 6, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Chaubey, N.; Yadav, D.K.; Singh, V.K.; Quraishi, M.A. A comparative study of leaves extracts for corrosion inhibition effect on aluminium alloy in alkaline medium. Ain Shams Eng. J. 2017, 8, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Abiola, O.K.; Otaigbe, J.O.E. The effects of Phyllanthus amarus extract on corrosion and kinetics of corrosion process of aluminum in alkaline solution. Corros. Sci. 2009, 51, 2790–2793. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- Gao, L.; Peng, S.; Gong, Z.; Chen, J. A combination of experiment and theoretical methods to study the novel and low-cost corrosion inhibitor 1-hydroxy-7-azabenzotriazole for mild steel in 1 M sulfuric acid. RSC Adv. 2018, 8, 38506–38516. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, P.; Su, L.; Fan, B.; Yan, F. One-step preparation and characterization of a Ce-La-Y ternary rare earth conversion coating on magnesium alloy AZ91D. Mater. Lett. 2021, 304, 130640. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Ebenso, E.E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Elsaeed, S.M.; El Tamany, E.S.H.; Ashour, H.; Zaki, E.G.; Khamis, E.A.; El Nagy, H.A. Corrosion and hydrogen evolution rate control for X-65 carbon steel based on chitosan polymeric ionic liquids: Experimental and quantum chemical studies. RSC Adv. 2018, 8, 37891–37904. [Google Scholar] [CrossRef] [Green Version]
- Lebrini, M.; Robert, F.; Lecante, A.; Roos, C. Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros. Sci. 2011, 53, 687–695. [Google Scholar] [CrossRef]
- Gadow, H.S.; Motawea, M.M.; Elabbasy, H.M. Investigation of myrrh extract as a new corrosion inhibitor for α-brass in 3.5% NaCl solution polluted by 16 ppm sulfide. RSC Adv. 2017, 7, 29883–29898. [Google Scholar] [CrossRef] [Green Version]
- Zehra, S.; Mobin, M.; Aslam, R.; Lgaz, H.; Chung, I.M. Assessment of biodegradable glycine and glutamic acid based ionic liquids as mild steel corrosion inhibitors in acid solution: An experimental and theoretical approach. J. Mol. Struct. 2021, 1240, 130505. [Google Scholar] [CrossRef]
- Alrebh, A.; Rammal, M.B.; Omanovic, S. A pyridine derivative 2-(2-Methylaminoethyl)pyridine (MAEP) as a ‘green’ corrosion inhibitor for low-carbon steel in hydrochloric acid media. J. Mol. Struct. 2021, 1238, 130333. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Bazzi, L.; El Issami, S. The role of pH in corrosion inhibition of tin using the proline amino acid: Theoretical and experimental investigations. RSC Adv. 2020, 10, 29696–29704. [Google Scholar] [CrossRef]
- Guo, L.; El Bakri, Y.; Anouar, E.H.; Tan, J.; Kaya, S.; Essassi, E.M. Multidimensional insights involving electrochemical andin silicoinvestigation into the corrosion inhibition of newly synthesized pyrazolotriazole derivatives on carbon steel in a HCl solution. RSC Adv. 2019, 9, 34761–34771. [Google Scholar] [CrossRef] [Green Version]
- El-Faham, A.; Osman, S.M.; Al-Lohedan, H.A.; El-Mahdy, G.A. Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution. Molecules 2016, 21, 714. [Google Scholar] [CrossRef] [Green Version]
- Umoren, S.A.; Li, Y.; Wang, F.H. Synergistic effect of iodide ion and polyacrylic acid on corrosion inhibition of iron in H2SO4 investigated by electrochemical techniques. Corros. Sci. 2010, 52, 2422–2429. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wang, F. An investigation of benzimidazole derivative as corrosion inhibitor for mild steel in different concentration HCl solutions. Corros. Sci. 2011, 53, 113–121. [Google Scholar] [CrossRef]
- Moumeni, O.; Chafaa, S.; Kerkour, R.; Benbouguerra, K.; Chafai, N. Synthesis, structural and anticorrosion properties of diethyl (phenylamino) methyl) phosphonate derivatives: Experimental and theoretical study. J. Mol. Struct. 2020, 1206, 127693. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Guo, L.; Kaya, C. Quantum chemical calculations, molecular dynamic (MD) simulations and experimental studies of using some azo dyes as corrosion inhibitors for iron. Part 2: Bis–azo dye derivatives. J. Mol. Struct. 2018, 1163, 397–417. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Wang, F. Inhibition Behavior of Ascorbic Benzoate for Steel Rebar in Alkaline Solution. Acta Chim. Sin. 2011, 69, 2359–2367. [Google Scholar]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. New coumarin derivative as an eco-friendly inhibitor of corrosion of mild steel in Acid medium. Molecules 2014, 20, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Abdulazeez, I.; Khaled, M.; Al-Saadi, A.A. Impact of electron-withdrawing and electron-donating substituents on the corrosion inhibitive properties of benzimidazole derivatives: A quantum chemical study. J. Mol. Struct. 2019, 1196, 348–355. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe(110) surface using the DFTB method. RSC Adv. 2017, 7, 29042–29050. [Google Scholar] [CrossRef] [Green Version]
- Kokalj, A. On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem. Phys. 2012, 393, 1–12. [Google Scholar] [CrossRef]
- El-Hendawy, M.M.; Kamel, A.M.; Mohamed, M.M.A. The anti-corrosive behavior of benzo-fused N-heterocycles: An in silico study toward developing organic corrosion inhibitors. Phys. Chem. Chem. Phys. 2022, 24, 743–756. [Google Scholar] [CrossRef]
- Stowasser, R.; Hoffmann, R. What Do the Kohn-Sham Orbitals and Eigenvalues Mean? J. Am. Chem. Soc. 1999, 14, 3414–3420. [Google Scholar] [CrossRef]
- Awad, M.K.; Mustafa, M.R.; Elnga, M.M.A. Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J. Mol. Struct. Theochem 2010, 959, 66–74. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, B.; Wang, M.; Shi, S.; Gong, L.; Gong, W.; Pang, H.; Liu, X.; Li, B.; Feng, Y.; et al. Chemically modified resveratrol as green corrosion inhibitor for Q235 steel: Electrochemical, SEM, UV and DFT studies. J. Mol. Liq. 2021, 343, 117672. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Sharma, P.; Guo, L.; Dagdag, O.; Kumar, V. Molecular dynamic simulation and Quantum chemical calculation of phytochemicals present in Beta vulgaris and electrochemical behaviour of Beta vulgaris peel extract as green corrosion inhibitor for stainless steel (SS-410) in acidic medium. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127707. [Google Scholar] [CrossRef]
- Derry, G.N.; Kern, M.E.; Worth, E.H. Recommended values of clean metal surface work functions. J. Vac. Sci. Technol. A 2015, 33, 060801. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Fatyeyeva, K. Ionic liquids as green and sustainable steel corrosion inhibitors: Recent developments Chem. Eng. J. 2021, 425, 131480. [Google Scholar] [CrossRef]
- Guimarães, T.A.S.; da Cunha, J.N.; de Oliveira, G.A.; da Silva, T.U.; de Oliveira, S.M.; de Araújo, J.R.; Machado, S.d.P.; D’Elia, E.; Rezende, M.J.C. Nitrogenated derivatives of furfural as green corrosion inhibitors for mild steel in HCl solution. J. Mater. Res. Technol. 2020, 9, 7104–7122. [Google Scholar] [CrossRef]
- Abbout, S.; Hsissou, R.; Erramli, H.; Chebabe, D.; Salim, R.; Kaya, S.; Hajjaji, N. Gravimetric, electrochemical and theoretical study, and surface analysis of novel epoxy resin as corrosion inhibitor of carbon steel in 0.5 M H2SO4 solution. J. Mol. Struct. 2021, 1245, 131014. [Google Scholar] [CrossRef]
- Sayin, K.; Karakaş, D. Quantum chemical studies on the some inorganic corrosion inhibitors. Corros. Sci. 2013, 77, 37–45. [Google Scholar] [CrossRef]
- Obot, I.B.; Kaya, S.; Kaya, C.; Tüzün, B. Density Functional Theory (DFT) modeling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide Schiff bases for steel corrosion. Physica E 2016, 80, 82–90. [Google Scholar] [CrossRef]
- Corzo, H.H.; Galano, A.; Dolgounitcheva, O.; Zakrzewski, V.G.; Ortiz, J.V. NR2 and P3+: Accurate, Efficient Electron-Propagator Methods for Calculating Valence, Vertical Ionization Energies of Closed-Shell Molecules. J. Phys. Chem. A 2015, 119, 8813–8821. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Martinez, S.; Valek, L.; Oslaković, I.S. Adsorption of Organic Anions on Low-Carbon Steel in Saturated Ca(OH)2 and the HSAB Principle. J. Electrochem. Soc. 2007, 154, C671–C677. [Google Scholar] [CrossRef]
- Durnie, W.; Marco, R.D.; Jefferson, A.; Kinsella, B. Development of a Structure-Activity Relationship for Oil Field Corrosion Inhibitors. J. Electrochem. Soc. 1999, 146, 1751–1756. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Lei, L.; Yang, H.; Zhu, Y. The applicaion of acid-base theory in corrosion processes. Corros. Sci. Prot. Technol. 2000, 12, 319–322. [Google Scholar]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Experimental and computational insights. J. Mol. Struct. 2021, 1227, 129374. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Guo, L.; Kaya, S.; Katin, K.P.; Verma, D.K.; Rbaa, M.; Dagdag, O.; Haldhar, R. Novel gossypol–indole modification as a green corrosion inhibitor for low–carbon steel in aggressive alkaline–saline solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128207. [Google Scholar] [CrossRef]
- Migahed, M.A.; Zaki, E.G.; Shaban, M.M. Corrosion control in the tubing steel of oil wells during matrix acidizing operations. RSC Adv. 2016, 6, 71384–71396. [Google Scholar] [CrossRef]
- Mazlan, N.; Jumbri, K.; Azlan Kassim, M.; Abdul Wahab, R.; Basyaruddin Abdul Rahman, M. Density functional theory and molecular dynamics simulation studies of bio-based fatty hydrazide-corrosion inhibitors on Fe (110) in acidic media. J. Mol. Liq. 2022, 347, 118321. [Google Scholar] [CrossRef]







| Cinh (g L−1) | βa (mV dec−1) | βc (mV dec−1) | icorr (mA cm−2) | Ecorr (mV) | IE (%) |
|---|---|---|---|---|---|
| 0 | 58 | 125 | 0.73 | −430 | / |
| 0.05 | 59 | 127 | 0.35 | −420 | 51.7 |
| 0.10 | 59 | 126 | 0.19 | −419 | 73.8 |
| 0.15 | 60 | 127 | 0.13 | −422 | 82.1 |
| 0.20 | 61 | 127 | 0.066 | −431 | 90.9 |
| 0.30 | 62 | 130 | 0.047 | −434 | 93.5 |
| Cinh (g L−1) | Rs (Ω cm2) | CPE−C (μF cm−2) | CPE−n | Rct (Ω cm2) | η (%) |
|---|---|---|---|---|---|
| 0 | 1.94 | 104 | 0.95 | 29.3 | / |
| 0.05 | 2.22 | 86.6 | 0.94 | 62.9 | 53.4 |
| 0.10 | 2.19 | 62.2 | 0.94 | 118 | 75.2 |
| 0.15 | 1.97 | 51.8 | 0.94 | 147 | 80.1 |
| 0.20 | 2.11 | 43.6 | 0.94 | 281 | 89.6 |
| 0.30 | 2.01 | 33.9 | 0.94 | 406 | 92.8 |
| Name of the Compound | Abbreviation | Retention Time (min) | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| 1-gala-l-ido-octose | GIO | 1.737 | C8H16O8 | 240 |
| Topotecan | TO | 2.215 | C23H23N3O5 | 421 |
| Styrene | ST | 8.616 | C8H8 | 104 |
| 2-ethyl-1-hexanol | EH | 13.14 | C8H18O | 130 |
| 2-amino-5-[(2-carboxy)vinyl]-imidazole | IACV | 15.24 | C6H7N3O2 | 153 |
| Adrenalone | AD | 19.62 | C9H11NO3 | 181 |
| Benzocycloheptatriene | BT | 22.02 | C11H10 | 142 |
| Actinobolin | AC | 24.73 | C13H20N2O6 | 300 |
| Pterin-6-carboxylic acid | PCA | 27.37 | C7H5N5O3 | 207 |
| 2,5-difluoro-β, 3, 4-trihydroxy-N-methyl-benzeneethanamine | BDTM | 30.74 | C8H11F2N | 219 |
| 4′-methyl-2-hydroxystilbene | MH | 33.26 | C15H14O | 210 |
| Compound | HOMO Distribution | LUMO Distribution |
|---|---|---|
| GIO | O | O |
| TO | Rings | Rings |
| ST | C in the branch | Rings |
| EH | Branch with O atom | Branch with O atom |
| IACV | Pentatomic ring, O, N | Pentatomic ring, O, N |
| AD | Benzene ring, N, O | Benzene ring, N, O |
| BT | Rings | Rings |
| AC | Rings, N | Rings, N |
| PCA | Rings, O | Rings, O |
| BDTM | O, N, Benzene ring | O, Benzene ring |
| MH | Benzene rings | Benzene rings |
| Compound | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | H (eV) | X (eV) | ΔN |
|---|---|---|---|---|---|---|
| GIO | −11.03 | 2.66 | 13.69 | 6.85 | 4.18 | 0.07 |
| TO | −8.17 | 1.09 | 9.25 | 4.63 | 3.54 | 0.17 |
| ST | −8.39 | 2.65 | 11.04 | 5.52 | 2.87 | 0.20 |
| EH | −11.33 | 3.74 | 15.07 | 7.53 | 3.79 | 0.08 |
| IACV | −7.80 | 2.35 | 10.14 | 5.07 | 2.72 | 0.23 |
| AD | −8.64 | 2.42 | 11.05 | 5.53 | 3.11 | 0.18 |
| BT | −8.09 | 2.60 | 10.69 | 5.35 | 2.75 | 0.22 |
| AC | −9.09 | 2.75 | 11.83 | 5.92 | 3.17 | 0.16 |
| PCA | −9.14 | 1.55 | 10.69 | 5.34 | 3.80 | 0.12 |
| BDTM | −8.64 | 3.58 | 12.21 | 6.10 | 2.53 | 0.21 |
| MH | −7.82 | 2.21 | 10.03 | 5.01 | 2.81 | 0.23 |
| Compound | Esurf+solu + Einh (kcal mol−1) | Etotal (kcal mol−1) | Eads (kcal mol−1) |
|---|---|---|---|
| TO | −30,302.11 | −31,204.32 | −902.21 |
| PCA | −30,444.21 | −31,331.86 | −887.65 |
| BDTM | −30,397.84 | −31,277.37 | −879.53 |
| AC | −30,430.67 | −31,246.72 | −816.05 |
| AD | −30,390.48 | −31,200.68 | −810.19 |
| ST | −30,001.78 | −30,759.53 | −757.75 |
| IACV | −30,570.80 | −31,195.94 | −625.14 |
| MH | −30,411.15 | −31,000.90 | −589.75 |
| BT | −30,353.40 | −30,924.26 | −570.86 |
| GIO | −30,351.34 | −30,850.19 | −498.85 |
| EH | −30,431.33 | −30,870.30 | −438.97 |
| Composition | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|
| Amount (%) | 0.16 | 0.14 | 0.48 | 0.03 | 0.03 | 99.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhang, S.; Hao, L.; Du, H.; Pan, R.; Huang, G.; Liu, H. Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules 2022, 27, 3826. https://doi.org/10.3390/molecules27123826
Feng L, Zhang S, Hao L, Du H, Pan R, Huang G, Liu H. Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules. 2022; 27(12):3826. https://doi.org/10.3390/molecules27123826
Chicago/Turabian StyleFeng, Lijuan, Shanshan Zhang, Long Hao, Hongchen Du, Rongkai Pan, Guofu Huang, and Haijian Liu. 2022. "Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis" Molecules 27, no. 12: 3826. https://doi.org/10.3390/molecules27123826