Efficient Oxidation of Cyclohexane over Bulk Nickel Oxide under Mild Conditions
Abstract
:1. Introduction
2. Results
2.1. Characterization of Bulk NiO
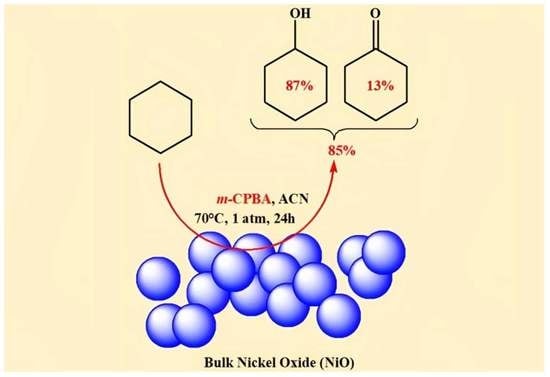
2.2. The Oxidation of Cyclohexane over NiO Powder
2.2.1. Effect of Oxidant
2.2.2. Effect of Reaction Temperature
2.2.3. Effect of Reaction Time
2.2.4. Effect of Catalyst Amount
2.3. Catalyst Recycling
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of NiO Powder
3.2.2. Characterization of NiO Powder
3.3. Oxidation of Cyclohexane over Bulk NiO
3.3.1. Molecular Oxygen (O2)
3.3.2. Using Hydrogen Peroxide (H2O2)
3.3.3. Tert-Butyl Hydroperoxide (TBHP)
3.3.4. Meta-Chloroperoxybenzoic Acid (m-CPBA)
3.3.5. The Optimization of the Oxidation of Cyclohexane over Bulk NiO Using m-CPBA
3.4. The Calculation of Conversion and Selectivity
3.5. Catalyst Recycling
3.6. Possible Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Aoki, M.; Noyori, R. A Green Route to Adipic Acid: Direct Oxidation of Cyclohexenes with 30% Hydrogen Peroxide. Science 1998, 281, 1646–1647. [Google Scholar] [CrossRef]
- Hwang, K.C.; Sagadevan, A. One-pot room-temperature conversion of cyclohexane to adipic acid by ozone and UV light. Science 2014, 346, 1495–1498. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes. Science 2019, 366, 1514–1517. [Google Scholar] [CrossRef]
- Deng, W.P.; Yan, L.F.; Wang, B.J.; Zhang, Q.H.; Song, H.Y.; Wang, S.S.; Zhang, Q.H.; Wang, Y. Efficient Catalysts for Green Synthesis of Adipic Acid from Biomass. Angew. Chem. Int. Ed. 2021, 60, 4712–4719. [Google Scholar] [CrossRef]
- Xu, L.; He, C.H.; Zhu, M.Q.; Wu, K.; Lai, Y. Silica-Supported Gold Catalyst Modified by Doping with Titania for Cyclohexane Oxidation. Catal. Lett. 2007, 118, 248–253. [Google Scholar] [CrossRef]
- Priyank, K.; Rajubhai, M. Review of a Cyclohexane Oxidation Reaction Using Heterogenous Catalyst. Int. J. Eng. Dev. Res. 2014, 2, 2321–9939. [Google Scholar]
- Xu, L.X.; He, C.H.; Zhu, M.Q.; Fang, S. A highly active Au/Al2O3 catalyst for cyclohexane oxidation using molecular oxygen. Catal. Lett. 2007, 114, 202–205. [Google Scholar] [CrossRef]
- Wu, P.; Bai, P.; Loh, K.P.; Zhao, X.S. Au nanoparticles dispersed on functionalized mesoporous silica for selective oxidation of cyclohexane. Catal. Today 2010, 158, 220–227. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Zheng, Y.; Chen, H.; Wang, F.; Ma, J. An Efficient and Reusable Catalyst of Bismuth-Containing SBA-15 Mesoporous Materials for Solvent-free Liquid Phase Oxidation of Cyclohexane by Oxygen. Catal. Lett. 2008, 122, 330–337. [Google Scholar] [CrossRef]
- Enache, D.; Carley, A.F.; Roberts, M.W.; Hutchings, G.J. Selective conversion of cyclohexane to cyclohexanol and cyclohexanone using a gold catalyst under mild conditions. Catal. Lett. 2005, 101, 175–179. [Google Scholar] [CrossRef]
- Abboud, M.; Alnefaie, R.; Alhanash, A. Unsupported and silica-supported nickel nanoparticles: Synthesis and application in catalysis. J. Nanopart. Res. 2022, 24, 21. [Google Scholar] [CrossRef]
- Jaji, N.-D.; Lee, H.L.; Hussin, M.H.; Akil, H.M.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotechnol. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Vollath, D.; Szabó, D.V. Nonmetallic bulk nanomaterials. In Bulk Nanostructured Materials; Zehetbauer, M.J., Zhu, Y.T., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009; pp. 49–85. [Google Scholar]
- Schubert, U.; Hüsing, N. Synthesis of Inorganic Materials, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–424. [Google Scholar]
- Ozin, G.A.; Arsenault, A.C.; Cademartiri, L. Chapter 5. Nanorod, Nanotube, Nanowire Self-Assembly. In Nanochemistry: A Chemical Approach to Nanomaterials; Royal Society of Chemistry: London, UK, 2009; pp. 215–330. [Google Scholar]
- Lai, T.L.; Lee, C.C.; Wu, K.S.; Shu, Y.Y.; Wang, C.B. Microwave-enhanced catalytic degradation of phenol over nickel oxide. Appl. Catal. B 2006, 68, 147–153. [Google Scholar] [CrossRef]
- Lai, T.L.; Lee, C.C.; Huang, G.L.; Shu, Y.Y.; Wang, C.B. Microwave-enhanced catalytic degradation of 4-chlorophenol over nickel oxides. Appl. Catal. B 2008, 78, 151–157. [Google Scholar] [CrossRef]
- Christoskova, S.T.; Stoyanova, M. Degradation of phenolic waste waters over Ni-oxide. Water Res. 2001, 35, 2073–2077. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Daud, W.W.; Hayashi, J.I. Governance of the porosity and of the methane decomposition activity sustainability of NiO/SiO2 nanocatalysts by changing the synthesis parameters in the modified Stöber method. C. R. Chim. 2017, 20, 896–909. [Google Scholar] [CrossRef]
- Guo, X.F.; Kim, Y.S.; Kim, G.J. Synthesis of Mesoporous Metal Oxides and Their Efficient Property for Super Capacitor Application. J. Nanosci. Nanotechnol. 2011, 11, 1672–1675. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Nieto, J.L.; Dejoz, A.; Cecilia, J.A.; Agouram, S.; Soriano, M.D.; Torres, V.; Jiménez-Jiménez, J.; Castellón, E.R. Nickel oxide supported on porous clay heterostructures as selective catalysts for the oxidative dehydrogenation of ethane. Catal. Sci. Technol. 2016, 6, 3419–3429. [Google Scholar] [CrossRef]
- Vikraman, D.; Park, H.J. Shape-selective synthesis of NiO nanostructures for hydrazine oxidation as a nonenzymatic amperometric sensor. RSC Adv. 2016, 6, 86101–86107. [Google Scholar] [CrossRef]
- Sasaki, T.; Ichikuni, N.; Hara, T.; Shimazu, S. Study on the promoting effect of nickel silicate for 1-phenylethanol oxidation on supported NiO nanocluster catalysts. Catal. Today 2018, 307, 29–34. [Google Scholar] [CrossRef]
- Gao, X.; Mao, H.; Lu, M.; Yang, J.; Li, B. Facile synthesis route to NiO–SiO2 intercalated clay with ordered porous structure: Intragallery interfacially controlled functionalization using nickel–ammonia complex for deep desulfurization. Microporous Mesoporous Mater. 2012, 148, 25–33. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Kiyozumi, Y.; Hanaoka, T.; Mizukami, F. Heterogeneous catalytic activity of NiO-silica composites designated with cubic Pm3n cage nanostructures. Appl. Catal. B 2008, 82, 169–179. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Kuniyil, M.; Khan, M.; Shaik, M.R.; Alwarthan, A.; Labis, J.P.; Siddiqui, M.R.H. Synthesis and comparative catalytic study of zinc oxide (ZnOx) nanoparticles promoted MnCO3, MnO2 and Mn2O3 for selective oxidation of benzylic alcohols using molecular oxygen. Mater. Express 2017, 7, 79–92. [Google Scholar] [CrossRef]
- Arora, A.K.; Kumar, P.; Kumar, S. Synthesis of ZnO Nanoparticle and its Application in Catalytic Hydrolysis of p-Acetoxynitrobenzene. Int. J. Nanosci. 2017, 16, 1750005. [Google Scholar] [CrossRef]
- Pike, S.D.; García-Trenco, A.; White, E.R.; Leung, A.H.; Weiner, J.; Shaffer, M.S.; Williams, C.K. Colloidal Cu/ZnO catalysts for the hydrogenation of carbon dioxide to methanol: Investigating catalyst preparation and ligand effects. Catal. Sci. Technol. 2017, 7, 3842–3850. [Google Scholar] [CrossRef] [Green Version]
- Chand, S.; Sandhu, J.S. ZnO Nanoparticles: An efficient green reusable catalyst for the synthesis of 3-formyl benzopyranones chalcones by Claisen-Schmidt reaction under solvent-free condition. Indian J. Chem. Sect. B 2015, 54, 1350–1354. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Cao, X.; Zhao, X. Optimization of Reaction Conditions for Cyclohexane to Cyclohexanone with t-Butylhydroperoxide Over CuCl2 Loaded with Activated Carbon. J. Braz. Chem. Soc. 2016, 27, 202–208. [Google Scholar]
- Abboud, M.; Haija, M.A.; Bel-Hadj-Tahar, R.; Mubarak, A.T.; Ismail, I.; Hamdy, M.S. Highly ordered mesoporous flower-like NiO nanoparticles: Synthesis, characterization and photocatalytic performance. New J. Chem. 2020, 44, 3402–3411. [Google Scholar] [CrossRef]
- Abboud, M. Immediate epoxidation of cyclohexene at room temperature using mesoporous flower-like NiO nanoparticles. React. Kinet. Mech. Catal. 2020, 131, 781–792. [Google Scholar] [CrossRef]
- Sahlabji, T.; Abboud, M.; Bel-Hadj-Tahar, R.; Hamdy, M.S. Spontaneous epoxidation of styrene catalyzed by flower-like NiO nanoparticles under ambient conditions. J. Nanopart. Res. 2020, 22, 364. [Google Scholar] [CrossRef]
- Grosso, C.; Boissiere, B.S.; Brezesinski, T.; Pinna, N.; Albouy, P.; Amenitsch, H.; Antonietti, M.; Sanchez, C. Periodically Ordered Nanoscale Islands and Mesoporous Films Composed of Nanocrystalline Multimetallic Oxides. Nat. Mater. 2004, 3, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Al-Zaqri, N.; Sahlabji, T.; Eissa, M.; Mubarak, A.T.; Bel-Hadj-Tahar, R.; Alsalme, A.; Alharthi, F.A.; Alsyahi, A.; Hamdy, M.S. Instant and quantitative epoxidation of styrene under ambient conditions over a nickel(ii)dibenzotetramethyltetraaza[14]annulene complex immobilized on amino-functionalized SBA-15. RSC Adv. 2020, 10, 35407–35418. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Sahlabji, T.; Eissa, M.; Bel-Hadj-Tahar, R.; Mubarak, A.T.; Al-Zaqri, N.; Hamdy, M.S. Nickel(II)dibenzotetramethyltetraaza[14]annulene complex immobilized on amino-functionalized TUD-1: An efficient catalyst for immediate and quantitative epoxidation of cyclohexene under ambient conditions. New J. Chem. 2020, 44, 20137–20147. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Al-Zaqri, N.; Sahlabji, T.; Eissa, M.; Abu Haija, M.; Alhanash, A.M.; Alsalme, A.; Alharthi, F.A.; Abboud, M. Instant Cyclohexene Epoxidation Over Ni-TUD-1 under Ambient Conditions. Catal. Lett. 2021, 151, 1612–1622. [Google Scholar] [CrossRef]
- Zou, X.; Conradsson, T.; Klingstedt, M.; Dadachov, M.S.; O’Keeffe, M. A mesoporous germanium oxide with crystalline pore walls and its chiral derivative. Nature 2005, 437, 716–719. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Y.; Tanabe, E.; Shishido, T.; Takehira, K. Carbon fibers prepared by pyrolysis of methane over Ni/MCM-41 catalyst. Microporous Mesoporous Mater. 2003, 57, 283–289. [Google Scholar] [CrossRef]
- Basha, S.S.; Sasirekha, N.R.; Maheswari, R.; Shanthi, K. Mesoporous H-AlMCM-41 supported NiO-MoO3 catalysts for hydrodenitrogenation of o-toluidine: I. Effect of MoO3 loading. Appl. Catal. A-Gen. 2006, 308, 91–98. [Google Scholar] [CrossRef]
- Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P.; Rodríguez-Castellón, E.; Jiménez-López, A. Nickel oxide supported on zirconium-doped mesoporous silica for selective catalytic reduction of NO with NH3. J. Mater. Chem. 2002, 12, 3331–3336. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Nanoparticle-supported and magnetically recoverable nickel catalyst: A robust and economic hydrogenation and transfer hydrogenation protocol. Green Chem. 2009, 11, 127–131. [Google Scholar] [CrossRef]
- Kalbasi, R.J.; Mosaddegh, N. Suzuki-Miyaura Cross-coupling Reaction Catalyzed by Nickel Nanoparticles Supported on Poly(N-vinyl-2-pyrrolidone)/TiO2-ZrO2 Composite. Bull. Korean Chem. Soc. 2011, 32, 2584–2592. [Google Scholar] [CrossRef] [Green Version]
- Alonso, F.; Riente, P.; Yus, M. Nickel nanoparticles in hydrogen transfer reactions. Acc. Chem. Res. 2011, 44, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Justin, P.; Ranga, R.G. Microwave-mediated synthesis for improved morphology and pseudocapacitance performance of nickel oxide. ACS Appl. Mater. Interfaces 2011, 3, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Xiao, B.; Du, L.J.; Yan, R.; Liang, T.D. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing cellulose. J. Fuel Chem. Technol. 2008, 36, 42–47. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Bimeghdar, S. Synthesis of mesoporous NiO nanoparticles and their application in the adsorption of Cr(VI). Chem. Eng. J. 2014, 239, 105–113. [Google Scholar] [CrossRef]
- Chen, M.M.; Coelho, P.S.; Arnold, F.H. Utilizing Terminal Oxidants to Achieve P450-Catalyzed Oxidation of Methane. Adv. Synth. Catal. 2012, 354, 964–968. [Google Scholar] [CrossRef] [Green Version]
- Kudrik, E.V.; Afanasiev, P.; Alvarez, L.X.; Dubourdeaux, P.; Clemancey, M.; Latour, J.M.; Blondin, G.; Bouchu, D.; Albrieux, F.; Nefedov, S.E.; et al. An N-bridged high-valent diiron-oxo species on a porphyrin platform that can oxidize methane. Nat. Chem. 2012, 4, 1024–1029. [Google Scholar] [CrossRef]
- Lyakin, O.Y.; Bryliakov, K.P.; Talsi, E.P. Non-heme oxoiron(V) intermediates in chemo-, regio- and stereoselective oxidation of organic substrates. Coord. Chem. Rev. 2019, 384, 126–139. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Hypervalent Iodine-Catalyzed Oxidative Functionalizations Including Stereoselective Reactions. Chem. Asian J. 2014, 9, 950–971. [Google Scholar] [CrossRef]
- Clavier, H.; Pellissier, H. Recent Developments in Enantioselective Metal-Catalyzed Domino Reactions. Adv. Synth. Catal. 2012, 354, 3347–3403. [Google Scholar] [CrossRef]
- Ghosh, P.; Ganguly, B.; Das, S. NaI/KI/NH4I and TBHP as powerful oxidation systems: Use in the formation of various chemical bonds. Org. Biomol. Chem. 2021, 19, 2146–2167. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Gong, J.L.; Qi, X.X. A powerful combination: Recent achievements on using TBAI and TBHP as oxidation system. Org. Biomol. Chem. 2014, 12, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Fokin, A.A.; Schreiner, P.R. Selective alkane transformations via radicals and radical cations: Insights into the activation step from experiment and theory. Chem. Rev. 2002, 102, 1551–1593. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Bjorsvik, H.R.; Fontana, F.; Minisci, F.; Serri, A. Radical versus “oxenoid” oxygen insertion mechanism in the oxidation of alkanes and alcohols by aromatic peracids. New synthetic developments. J. Org. Chem. 1996, 61, 9409–9416. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Green, I.R.; Ahmed, I.; Abbas, G.; Rehman, N.U. Meta-Chloroperbenzoic acid (mCPBA): A versatile reagent in organic synthesis. RSC Adv. 2014, 4, 12882–12917. [Google Scholar] [CrossRef]
- Smith, J.G. The Review of high pressure science and technology 7, 1250–1252, 1998. Synth. Useful React. Epoxides 1984, 629, 656. [Google Scholar] [CrossRef]
- Parker, R.-E.; Isaacs, N.S. Mechanisms of Epoxide Reactions. Chem. Rev. 1959, 198459, 737–799. [Google Scholar] [CrossRef]
- Kooti, M.; Afshari, M. Phosphotungstic acid supported on magnetic nanoparticles as an efficient reusable catalyst for epoxidation of alkenes. Mater. Res. Bull. 2012, 47, 3473–3478. [Google Scholar] [CrossRef]
- Dmytro, S.N.; Oksana, V.N. Catalytic Oxidations with Meta-Chloroperoxybenzoic Acid (m-CPBA) and Mono- and Polynuclear Complexes of Nickel: A Mechanistic Outlook. Catalysts 2021, 11, 1148. [Google Scholar] [CrossRef]












| Catalyst Dose (mg) | Temperature (°C) | Reaction Time (hours) |
|---|---|---|
| 5, 10, 30, 50 | 25, 40, 60, 70 | 0.5, 1, 2, 4, 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnefaie, R.S.; Abboud, M.; Alhanash, A.; Hamdy, M.S. Efficient Oxidation of Cyclohexane over Bulk Nickel Oxide under Mild Conditions. Molecules 2022, 27, 3145. https://doi.org/10.3390/molecules27103145
Alnefaie RS, Abboud M, Alhanash A, Hamdy MS. Efficient Oxidation of Cyclohexane over Bulk Nickel Oxide under Mild Conditions. Molecules. 2022; 27(10):3145. https://doi.org/10.3390/molecules27103145
Chicago/Turabian StyleAlnefaie, Reem S., Mohamed Abboud, Abdullah Alhanash, and Mohamed S. Hamdy. 2022. "Efficient Oxidation of Cyclohexane over Bulk Nickel Oxide under Mild Conditions" Molecules 27, no. 10: 3145. https://doi.org/10.3390/molecules27103145