Contributions of Microelectrochemical Scanning Techniques for the Efficient Detection of Localized Corrosion Processes at the Cut Edges of Polymer-Coated Galvanized Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
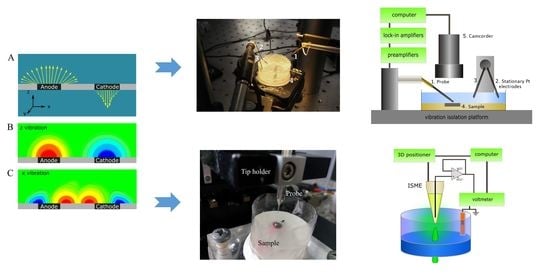
2.2. Scanning Microelectrochemical Measurements
3. Results and Discussion
3.1. Current Density Distributions Measured over the Cut Edge Systems by SVET
3.2. pH Line Scans Measured over the Cut Edge Systems
3.3. pH Quasi-Maps of the Cut Edge Systems
3.4. Measurements over the Cut Edge Systems Using a Zn ISME
3.5. Simultaneous Measurements with the Zn ISME-Sb Double-Barrel Microelectrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grundmeier, G.; Simões, A. Corrosion protection by organic coatings. In Encyclopedia of Electrochemistry, Volume 4: Corrosion and Oxide Films; Stratmann, M., Frankel, G.S., Eds.; Wiley−VCH: Weinheim, Germany, 2003; pp. 500–566. [Google Scholar]
- Soleymanibrojeni, M.; Shi, H.; Udoh, I.I.; Liu, F.; Han, E.-H. Microcontainers with 3-imino-1,2,4-triazole-5-thiolfor enhancing anticorrosión waterborne coatings for AA2024-T3. Prog. Org. Coat. 2019, 137, 105335. [Google Scholar]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Lillard, R.S. Scanning electrode techniques for investigating near-surface solution current densities. In Analytical Methods in Corrosion Science and Engineering; Marcus, P., Mansfeld, F., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 571–604. [Google Scholar]
- Bastos, A.C.; Quevedo, M.C.; Karavai, O.V.; Ferreira, M.G.S. Review—on the application of the scanning vibrating electrode technique (SVET) to corrosion research. J. Electrochem. Soc. 2017, 164, C973–C990. [Google Scholar] [CrossRef]
- Niu, L.; Yin, Y.; Guo, W.; Lu, M.; Qin, R.; Chen, S. Application of scanning electrochemical microscope in the study of corrosion of metals. J. Mater. Sci. 2009, 44, 4511–4521. [Google Scholar] [CrossRef]
- Jensen, M.B.; Tallman, D.E. Application of SECM to corrosion studies. In Electroanalytical Chemistry: A Series of Advances; Bard, A.J., Zoski, C., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Volume 24, pp. 171–286. [Google Scholar]
- Payne, N.A.; Stephens, L.I.; Mauzeroll, J. The application of scanning electrochemical microscopy to corrosion research. Corrosion 2017, 73, 759–780. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Wu, S.-L.; Orazem, M.E.; Pebere, N.; Tribollet, B.; Vivier, V. Local electrochemical impedance spectroscopy: A review and some recent developments. Electrochim. Acta 2011, 56, 8048–8057. [Google Scholar] [CrossRef] [Green Version]
- Worsley, D.; McMurray, H.N.; Belghazi, A. Determination of localised corrosion mechanisms using a scanning vibrating reference electrode technique. Chem. Commun. 1997, 2369–2370. [Google Scholar] [CrossRef]
- Bohm, S.; McMurray, H.N.; Powell, S.M.; Worsley, D. Photoelectrochemical investigation of corrosion using scanning electrochemical techniques. Electrochim. Acta 2000, 45, 2165–2174. [Google Scholar] [CrossRef]
- Worsley, D.A.; Williams, D.; Ling, J.S.G. Mechanistic changes in cut edge corrosion induced by variation of organic coating porosity. Corros. Sci. 2001, 43, 2335–2348. [Google Scholar] [CrossRef]
- Elvins, J.; Spittle, J.A.; Sullivan, J.H.; Worsley, D.A. The effect of magnesium additions on the microstructure and cut edge corrosion resistance of zinc aluminium alloy galvanised steel. Corros. Sci. 2008, 50, 1650–1658. [Google Scholar] [CrossRef]
- Ogle, K.; Baudu, V.; Garrigues, L.; Philippe, X. Localized electrochemical methods applied to cut edge corrosion. J. Electrochem. Soc. 2000, 147, 3654–3660. [Google Scholar] [CrossRef]
- Ogle, K.; Morel, S.; Jacquet, D. Observation of self-healing functions on the cut edge of galvanized steel using SVET and pH microscopy. J. Electrochem. Soc. 2006, 153, B1–B5. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Investigation of self-healing mechanism on galvanized steels cut edges by coupling SVET and numerical modelling. Electrochim. Acta 2008, 53, 5226–5234. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Modeling bimetallic corrosion under thin electrolytes. Corros. Sci. 2011, 53, 201–207. [Google Scholar] [CrossRef]
- Bastos, A.C.; Simões, A.M.; Ferreira, M.G. Corrosion of electrogalvanized steel in 0.1 M NaCl studied by SVET. Port. Electrochim. Acta 2003, 21, 371–387. [Google Scholar] [CrossRef]
- Simões, A.M.; Torres, J.; Picciochi, R.; Fernandes, J.C.S. Corrosion inhibition at galvanized steel cut edges by phosphate pigments. Electrochim. Acta 2009, 54, 3857–3865. [Google Scholar] [CrossRef]
- Custodio, J.V.; Agostinho, S.M.L.; Simões, A.M.P. Electrochemistry and surface analysis of the effect of benzotriazole on the cut edge corrosion of galvanized steel. Electrochim. Acta 2010, 55, 5523–5531. [Google Scholar] [CrossRef]
- Simões, A.M.; Fernandes, J.C.S. Studying phosphate corrosion inhibition at the cut edge of coil coated galvanized steel using the SVET and EIS. Prog. Org. Coat. 2010, 69, 219–224. [Google Scholar] [CrossRef]
- Alvarez-Pampliega, A.; Lamaka, S.V.; Taryba, M.G.; Madari, M.; De Strycker, J.; Tourwé, E.; Ferreira, M.G.S.; Terryn, H. Cut edge corrosion study on painted aluminum rich metallic coated steel by scanning vibrating electrode and micro-potentiometric techniques. Electrochim. Acta 2012, 61, 107–117. [Google Scholar] [CrossRef]
- Marques, A.G.; Izquierdo, J.; Souto, R.M.; Simões, A.M. SECM imaging of the cut edge corrosion of galvanized steel as a function of pH. Electrochim. Acta 2015, 153, 238–245. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Taryba, M.; Montemor, M.F.; Isaacs, H.S.; Ferreira, M.G.S. Quasi-simultaneous measurements of ionic currents by vibrating probe and pH distribution by ion-selective microelectrode. Electrochem. Commun. 2011, 13, 20–23. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Souto, R.M.; Ferreira, M.G.S. In-situ visualization of local corrosion by scanning ion-selective electrode technique (SIET). In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex Research Center: Badajoz, Spain, 2010; Volume 3, pp. 2162–2173. [Google Scholar]
- Thomas, S.; Izquierdo, J.; Birbilis, N.; Souto, R.M. Possibilities and limitations of scanning electrochemical microscopy of Mg and Mg alloys. Corrosion 2015, 71, 171–183. [Google Scholar] [CrossRef]
- Eckhard, K.; Chen, X.X.; Turcu, F.; Schuhmann, W. Redox competition mode of scanning electrochemical microscopy (RC-SECM) for visualisation of local catalytic activity. Phys. Chem. Chem. Phys. 2006, 8, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.M.; Fernández-Mérida, L.; González, S. SECM imaging of interfacial processes in defective organic coatings applied on metallic substrates using oxygen as redox mediator. Electroanalysis 2009, 21, 2640–2646. [Google Scholar] [CrossRef]
- González-García, Y.; Mol, J.M.C.; Muselle, T.; De Graeve, I.; Van Assche, G.; Scheltjens, G.; Van Mele, B.; Terryn, H. SECM study of defect repair in self-healing polymer coatings on metals. Electrochem. Commun. 2011, 13, 169–173. [Google Scholar] [CrossRef]
- Souto, R.M.; González-García, Y.; Battistel, D.; Daniele, S. In situ scanning electrochemical microscopy (SECM) detection of metal dissolution during zinc corrosion by means of mercury sphere-cap microelectrode tips. Chem. Eur. J. 2012, 18, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pérez, B.M.; Izquierdo, J.; González, S.; Souto, R.M. Scanning electrochemical microscopy studies for the characterization of localized corrosion reactions at cut edges of coil-coated steel. J. Solid State Electrochem. 2014, 18, 2983–2992. [Google Scholar] [CrossRef]
- Fernández-Pérez, B.M.; Izquierdo, J.; Santana, J.J.; González, S.; Souto, R.M. Scanning electrochemical microscopy studies for the characterization of localized corrosion reactions at cut edges of painted galvanized steel as a function of solution pH. Int. J. Electrochem. Sci. 2015, 10, 10145–10156. [Google Scholar]
- Lamaka, S.V.; Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Ferreira, M.G.S. Monitoring local spatial distribution of Mg2+, pH and ionic currents. Electrochem. Commun. 2008, 10, 259–262. [Google Scholar] [CrossRef]
- Souto, R.M.; Izquierdo, J.; Santana, J.J.; Kiss, A.; Nagy, L.; Nagy, G. Progress in scanning electrochemical microscopy by coupling potentiometric and amperometric measurement modes. In Current Microscopy Contributions to Advances in Science and Technology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2012; Volume 2, pp. 1407–1415. [Google Scholar]
- Izquierdo, J.; Nagy, L.; Varga, Á.; Bitter, I.; Nagy, G.; Souto, R.M. Scanning electrochemical microscopy for the investigation of corrosion processes: Measurement of Zn2+ spatial distribution with ion selective microelectrodes. Electrochim. Acta 2012, 59, 398–403. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Izquierdo, J.; Nagy, L.; Nagy, G.; Souto, R.M. Combined amperometric/potentiometric probes for improved chemical imaging of corroding surfaces using scanning electrochemical microscopy. Electrochim. Acta 2016, 221, 48–55. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Izquierdo, J.; Kiss, A.; Nagy, L.; Nagy, G.; Souto, R.M. Improved potentiometric SECM imaging of galvanic corrosion reactions. Corros. Sci. 2017, 129, 136–145. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Kiss, A.; Nagy, L.; Nagy, G.; Souto, R.M. Double barrel microelectrode assembly to prevent electrical field effects in potentiometric SECM imaging of galvanic corrosion processes. J. Electrochem. Soc. 2018, 165, C270–C277. [Google Scholar] [CrossRef] [Green Version]
- Filotás, D.; Fernández-Pérez, B.M.; Nagy, L.; Nagy, G.; Souto, R.M. Multi-barrel electrodes containing an internal micro-reference for the improved visualization of the galvanic corrosion of magnesium using potentiometric SECM. Sens. Actuat. B Chem. 2019, 296, 126625. [Google Scholar] [CrossRef]
- Souto, R.M.; Scantlebury, D.J. Cathodic delamination of coil coatings produced with different Zn-based intermediate metallic layers. Prog. Org. Coat. 2005, 53, 63–70. [Google Scholar] [CrossRef]
- Souto, R.M.; Fernández-Mérida, L.; González, S.; Scantlebury, D.J. Comparative EIS study of different Zn-based intermediate metallic layers in coil-coated steels. Corros. Sci. 2006, 48, 1182–1192. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Izquierdo, J.; Nagy, L.; Nagy, G.; Souto, R.M. Novel dual microelectrode probe for the simultaneous visualization of local Zn2+ and pH distributions in galvanic corrosion processes. Corros. Sci. 2017, 114, 37–44. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; Varga, Á.; Santana, J.J.; Nagy, G.; Souto, R.M. Spatially resolved measurement of electrochemical activity and pH distributions in corrosion processes by scanning electrochemical microscopy using antimony microelectrode tips. Electrochim. Acta 2011, 56, 8846–8850. [Google Scholar] [CrossRef]
- Varga, Á.; Nagy, L.; Izquierdo, J.; Bitter, I.; Souto, R.M.; Nagy, G. Development of solid contact micropipette Zn-ion selective electrode for corrosion studies. Anal. Lett. 2011, 44, 2876–2886. [Google Scholar] [CrossRef]
- Thomas, S.; Birbilis, N.; Venkatraman, M.S.; Cole, I.S. Corrosion of zinc as a function of pH. Corrosion 2012, 68, 015009. [Google Scholar] [CrossRef]
- Kiss, A.; Filotás, D.; Souto, R.M.; Nagy, G. The effect of electric field on potentiometric scanning electrochemical microscopic imaging. Electrochem. Commun. 2017, 77, 138–141. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filotás, D.; Izquierdo, J.; Fernández-Pérez, B.M.; Nagy, L.; Nagy, G.; Souto, R.M. Contributions of Microelectrochemical Scanning Techniques for the Efficient Detection of Localized Corrosion Processes at the Cut Edges of Polymer-Coated Galvanized Steel. Molecules 2022, 27, 2167. https://doi.org/10.3390/molecules27072167
Filotás D, Izquierdo J, Fernández-Pérez BM, Nagy L, Nagy G, Souto RM. Contributions of Microelectrochemical Scanning Techniques for the Efficient Detection of Localized Corrosion Processes at the Cut Edges of Polymer-Coated Galvanized Steel. Molecules. 2022; 27(7):2167. https://doi.org/10.3390/molecules27072167
Chicago/Turabian StyleFilotás, Dániel, Javier Izquierdo, Bibiana M. Fernández-Pérez, Lívia Nagy, Géza Nagy, and Ricardo M. Souto. 2022. "Contributions of Microelectrochemical Scanning Techniques for the Efficient Detection of Localized Corrosion Processes at the Cut Edges of Polymer-Coated Galvanized Steel" Molecules 27, no. 7: 2167. https://doi.org/10.3390/molecules27072167