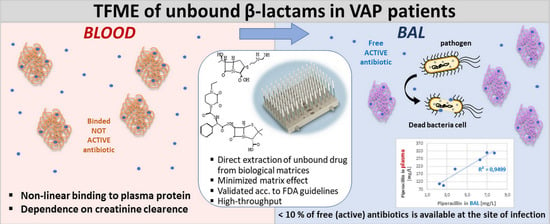
Quantitative Determination of Unbound Piperacillin and Imipenem in Biological Material from Critically Ill Using Thin-Film Microextraction-Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. LC-MS Conditions Optimization
2.1.1. Chromatographic Column Selection
2.1.2. Mobile Phase Gradient Selection
2.1.3. MS Conditions Optimization
2.1.4. Quality Control of TFME Blades Preparation
2.2. TFME Procedure Optimization
2.2.1. Solid Phase and Desorption Solvent Selection
2.2.2. Extraction and Desorption Time
2.3. Method Validation
2.3.1. Linearity Range
2.3.2. LLOQ and Sensitivity
2.3.3. Accuracy and Precision
2.3.4. Selectivity and Specificity
2.3.5. Carryover
2.3.6. Recovery
2.4. Application in Biological Samples
2.4.1. Patient Characteristics
2.4.2. Concentration of Piperacillin and Imipenem in Plasma
2.4.3. Degree of Penetration of Piperacillin and Imipenem to BAL
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.2.1. Optimization of LC-MS Conditions
3.2.2. Final LC-MS Conditions
3.2.3. Preparation of TFME Coating
3.2.4. Quality Control of TFME Blades
3.3. Optimization of TFME Procedure
3.4. Method Validation
3.4.1. Linearity Range
3.4.2. LLOQ and Sensitivity
3.4.3. Accuracy and Precision
3.4.4. Selectivity and Specificity
3.4.5. Carry-Over
3.4.6. Recovery
3.5. Blood and BAL Sampling from VAP Patients
4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mirtalaei, N.; Farazi, A.; Ebrahimi Monfared, M.; Jokar, A. Efficacy of antibiotic prophylaxis against ventilator-associated pneumonia. J. Hosp. Infect. 2019, 101, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Park, D.R. Microbiology of Ventilator-Associated Pneumonia. Respir. Care 2005, 50, 742–765. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; Sistla, S.; Dutta, T.K.; Badhe, A.S.; Rasitha, D.; Parija, S.C. Ventilator-associated pneumonia in a tertiary care hospital in India: Role of multi-drug resistant pathogens. J. Infect. Dev. Ctries. 2010, 4, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaoglan, H.; Yalcin, A.N.; Cengiz, M.; Ramazanoglu, A.; Ogunc, D.; Erbay, R.H.; Yilmaz, M.; Mamikoglu, L. Cost analysis of ventilator-associated pneumonia in Turkish medical-surgical intensive care units. Infez. Med. 2010, 4, 248–255. [Google Scholar]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Hons, B.P.; Kruger, P.; Paterson, D.L.; Lipman, J. Antibiotic resistance—What’s dosing got to do with it? Crit. Care Med. 2008, 36, 2433–2440. [Google Scholar] [CrossRef]
- Chastre, J.; Wunderink, R.; Prokocimer, P.; Lee, M.; Kaniga, K.; Friedland, I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: A multicenter, randomized study. Crit. Care Med. 2008, 36, 1089–1096. [Google Scholar] [CrossRef]
- Hayashi, Y.; Roberts, J.A.; Paterson, D.L.; Lipman, J. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1017–1031. [Google Scholar] [CrossRef]
- Felton, T.W.; McCalman, K.; Malagon, I.; Isalska, B.; Whalley, S.; Goodwin, J.; Bentley, A.M.; Hope, W.W. Pulmonary penetration of piperacillin and tazobactam in critically ill patients. Clin. Pharmacol. Ther. 2014, 96, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Tegeder, I.; Schmidtko, A.; Bräutigam, L.; Kirschbaum, A.; Geisslinger, G.; Lötsch, J. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 2002, 71, 325–333. [Google Scholar] [CrossRef]
- Zander, J.; Döbbeler, G.; Nagel, D.; Scharf, C.; Huseyn-Zada, M.; Jung, J.; Frey, L.; Vogeser, M.; Zoller, M. Variability of piperacillin concentrations in relation to tazobactam concentrations in critically ill patients. Int. J. Antimicrob. Agents 2016, 48, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Papaioannou, O.; Kaponi, M.; Kozanidou, A.; Hillas, G.; Stavropoulou, E.; Bouros, D.; Dimakou, K. Low penetrance of antibiotics in the epithelial lining fluid. The role of inhaled antibiotics in patients with bronchiectasis. Pulm. Pharmacol. Ther. 2020, 60, 101885. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein binding of β-lactam antibiotics in critically Ill patients: Can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hites, M.; Taccone, F.S.; Wolff, F.; Cotton, F.; Beumier, M.; De Backer, D.; Roisin, S.; Lorent, S.; Surin, R.; Seyler, L.; et al. Case-control study of drug monitoring of β-lactams in obese critically ill patients. Antimicrob. Agents Chemother. 2013, 57, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuner, E.A.; Ahrens, C.L.; Groszek, J.J.; Isada, C.; Vogelbaum, M.A.; Fissell, W.H.; Bhimraj, A. Use of therapeutic drug monitoring to treat Elizabethkingia meningoseptica meningitis and bacteraemia in an adult. J. Antimicrob. Chemother. 2012, 67, 1558–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Ulldemolins, M.; Roberts, M.S.; Mcwhinney, B.; Ungerer, J.; Paterson, D.L.; Lipman, J. International Journal of Antimicrobial Agents Therapeutic drug monitoring of β-lactams in critically ill patients: Proof of concept. Int. J. Antimicrob. Agents 2010, 36, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Giannella, M.; Lewis, R.; Viale, P.; Petrosillo, N. Treatment of carbapenem-resistant Klebsiella pneumoniae: The state of the art. Expert Rev. Anti-Infect. Therapy 2014, 11, 159–177. [Google Scholar] [CrossRef]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demoré, B.; Dellamonica, J.; Pulcini, C. The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Denooz, R.; Charlier, C. Simultaneous determination of five β-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 2008, 864, 161–167. [Google Scholar] [CrossRef]
- Verdier, M.-C.; Tribut, O.; Tattevin, P.; Le Tulzo, Y.; Michelet, C. Simultaneous Determination of 12 β-Lactam Antibiotics in Human Plasma by High-Performance Liquid Chromatography with UV Detection: Application to Therapeutic Drug Monitoring. Antimicrob. Agents Chemother. 2011, 55, 4873–4879. [Google Scholar] [CrossRef] [Green Version]
- Wolff, F.; Deprez, G.; Seyler, L.; Taccone, F.; Hites, M.; Gulbis, B.; Vincent, J.-L.; Jacobs, F.; Cotton, F. Talanta Rapid quantification of six b -lactams to optimize dosage regimens in severely septic patients. N. Engl. J. Med. 2013, 103, 153–160. [Google Scholar] [CrossRef]
- Gu, G.; Black, M.; Cookson, C.; Fiorella, A.; Li, Y.; Gorman, S.H.; Bakhtiar, R. Validation of an LC-MS/MS method for simultaneous quantification of venlafaxine and its five metabolites in rat plasma and its application in a pharmacokinetic study. J. Chromatogr. B 2018, 1087, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Naicker, S.; Valero, Y.C.G.; Meija, J.L.O.; Lipman, J.; Roberts, J.A.; Ord, L.; Wallis, C.; Parker, S.L. A UHPLC-MS/MS method for the simultaneous determination of piperacillin and tazobactam in plasma (total and unbound), urine and renal replacement therapy effluent. J. Pharm. Biomed. Anal. 2017, 148, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Najjar, N.; Jantsch, J.; Gessner, A. The use of liquid chromatography-tandem mass spectrometry for therapeutic drug monitoring of antibiotics in cancer patients. Clin. Chem. Lab. Med. 2017, 55, 1246–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barco, S.; Bandettini, R.; Maffia, A.; Tripodi, G.; Cangemi, G. Quantification of piperacillin, tazobactam, meropenem, ceftazidime, and linezolid in human plasma by liquid chromatography/tandem mass spectrometry. J. Chemother. 2015, 27, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.; Sun, H.; Chen, G.; Lin, S.; Kuo, C. Talanta Simultaneous quantification of antimicrobial agents for multidrug-resistant bacterial infections in human plasma by ultra-high-pressure liquid chromatography-tandem mass spectrometry. Talanta 2013, 116, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction, 1st ed.; Chemical Industry Press of China: Beijing, China, 2012; ISBN 9780124160170. [Google Scholar]
- Filipiak, W.; Bojko, B. SPME in clinical, pharmaceutical, and biotechnological research-how far are we from daily practice? TrAC Trends Anal. Chem. 2019, 205, 203–213. [Google Scholar] [CrossRef]
- US Food and Drug Administration Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. (accessed on 9 November 2020).
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; Van Belkum, A.; Caniaux, I. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol Infect. Dis 2019, 39, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Legrand, T.; Vodovar, D.; Tournier, N.; Khoudour, N. Simultaneous Determination of Eight β-Lactam Antibiotics, Performance Liquid Chromatography with Ultraviolet Detection. J. Food Drug Analys. 2016, 60, 4734–4742. [Google Scholar] [CrossRef]
- Nemutlu, E.; Kır, S.; Katlan, D.; Beksac, M.S. Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: Application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta 2009, 80, 117–126. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. J. Chromatogr. Sci. 2000, 38, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojko, B.; Mirnaghi, F.; Pawliszyn, J. Solid-phase microextraction: A multi-purpose microtechnique. Bioanalysis 2011, 3, 1895–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfizer Ltd. Tazocin 4 g/0.5 g Powder for Solution for Infusion. Electron. Med. Compend. 2013, pp. 1–12. Available online: http://www.medicines.org.uk/emc/medicine/28280 (accessed on 22 September 2021).
- SPC Primaxin IV 500 mg/500 mg Powder for Solution for Infusion. 2018, pp. 1–12. Available online: https://www.medicines.org.uk/emc/product/1515 (accessed on 22 September 2021).






| Time (min) | % A Phase | % B Phase |
|---|---|---|
| 0.01 | 95.00 | 5.00 |
| 0.20 | 95.00 | 5.00 |
| 1.00 | 60.00 | 40.00 |
| 2.00 | 60.00 | 40.00 |
| 3.50 | 5.00 | 95.00 |
| 5.00 | 5.00 | 95.00 |
| 5.01 | 95.00 | 5.00 |
| 7.20 | 95.00 | 5.00 |
| Compounds | Parent Ion m/z | Product Ions m/z | Collision Energy (V) | Q1 (V) | Q3 (V) | Retention Time (min) |
|---|---|---|---|---|---|---|
| Imipenem | 300.00 | 141.85 | −28.00 | −10.00 | −14.00 | 1.76 |
| 98.05 | −28.00 | −10.00 | −19.00 | |||
| 125.95 | −20.00 | −13.00 | −19.00 | |||
| Piperacillin | 518.05 | 143.00 | −20.00 | −22.00 | −15.00 | 2.53 |
| 159.95 | −19.00 | −22.00 | −30.00 | |||
| 359.00 | −10.00 | −22.00 | −12.00 |
| Compounds | Concentration [mg/L] | Within-Run [%] | Between-Run [%] | ||
|---|---|---|---|---|---|
| 1st Run | 2nd Run | 3rd Run | |||
| Imipenem | 0.05 | 110.54–115.53 | 109.07–112.82 | 88.52–92.87 | 88.52–115.53 |
| 0.15 | 102.02–112.09 | 99.88–106.51 | 88.60–108.93 | 88.60–112.09 | |
| 0.5 | 85.19–98.33 | 71.47–92.74 | 94.83–102.00 | 71.47–102.00 | |
| 1.0 | 91.68–100.12 | 93.65–86.91 | 97.33–107.89 | 86.91–107.89 | |
| Piperacillin | 0.02 | 83.26–118.56 | 83.93–113.37 | 78.14–100.68 | 79.14–118.56 |
| 0.06 | 88.25–111.57 | 90.36–111.57 | 81.82–116.08 | 81.82–116.08 | |
| 0.25 | 96.13–117.56 | 88.65–107.25 | 86.49–102.08 | 88.65–117.56 | |
| 0.5 | 95.15–103.52 | 85.08–103.78 | 92.68–99.15 | 85.08–103.78 | |
| Compounds | Concentration [mg/L] | Within-Run [%] | Between-Run [%] | ||
|---|---|---|---|---|---|
| 1st Run | 2nd Run | 3rd Run | |||
| Imipenem | 0.05 | 1.81 | 1.35 | 2.06 | 10.59 |
| 0.15 | 3.67 | 2.77 | 9.05 | 6.91 | |
| 0.5 | 5.90 | 10.49 | 2.79 | 11.48 | |
| 1.0 | 3.38 | 2.72 | 4.54 | 6.47 | |
| Piperacillin | 0.02 | 14.73 | 13.12 | 11.51 | 13.43 |
| 0.06 | 10.43 | 9.32 | 10.38 | 10.72 | |
| 0.25 | 9.29 | 8.09 | 7.64 | 9.74 | |
| 0.5 | 3.38 | 7.81 | 2.87 | 5.08 | |
| Compounds | Peak Area for Blank | Peak Area for LLOQ | % of LLOQ |
|---|---|---|---|
| Imipenem | 4.40 × 103 | 2.09 × 105 | 2.10% |
| 7.73 × 102 | 0.37% | ||
| 3.24 × 103 | 1.55% | ||
| 4.05 × 103 | 1.94% | ||
| Piperacillin | 2.82 × 104 | 6.84 × 105 | 4.13% |
| 8.61 × 103 | 1.26% | ||
| 1.56 × 104 | 2.28% | ||
| 2.77 × 104 | 4.05% |
| Pat. # | Age [years] | Body Weight [kg] | Microorganisms Found | Other Drugs | Renal Impairment Therapy | PCT [ng/mL] | CRP [mg/L] | Albumins [g/L] | Urea [mmol/L] | Creatinine [µmol/L] |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients receiving PIPERACILLIN | ||||||||||
| 1 | 89 | 88 | P. aeruginosa, P. mirabilis, Enterococcus spp. | - | - | 1.27 | 88.69 | 34.68 | 9.4 | 82.06 |
| 2 | 28 | 78 | P. aeruginosa, S. marcescens | - | - | 0.13 | 35.66 | 33.1 | 11.6 | 29.36 |
| 3 | 60 | 71 | P. mirabilis, E. coli | - | - | 1.4 | 69.56 | 29.1 | 5.3 | 61.71 |
| - | 0.69 | 51.08 | - | 4.3 | 61.2 | |||||
| - | 0.39 | 53.65 | 32.54 | 3.6 | 54.36 | |||||
| 4 | 73 | 64 | - | - | - | 0.59 | 76.48 | 29.37 | 8.6 | 50.87 |
| 0.45 | 42.08 | 28.99 | 9.3 | 55.67 | ||||||
| 5 | 83 | 82 | - | - | - | 0.3 | 133.56 | 24.9 | 13.5 | 82.31 |
| 6 | 66 | 86 | P. mirabilis, E. faecalis | - | CVVHD | 0.67 | 179.61 | - | 10.3 | 96.98 |
| 7 | 64 | 88 | E. coli | - | - | 0.19 | 109.19 | 29.89 | 4.1 | 54.7 |
| 8 | 69 | 86 | E. coli, Candida spp. | - | - | 0.06 | 60.14 | 31.64 | 4 | 28.72 |
| 9 | 68 | 68 | S. hominis | - | - | 0.15 | 6.26 | 37.67 | 3 | 46.89 |
| 10 | 46 | 76 | K. pneumoniae | - | - | 0.28 | 184.35 | 32.86 | 10.1 | 53.53 |
| 11 | 77 | 62 | C. albicans | - | - | 2.37 | 195.97 | 28.58 | 13.6 | 105.33 |
| Patients receiving IMIPENEM | ||||||||||
| 1 | 71 | 83 | E.coli | piperacillin | diuresis supported with FSD | 24.39 | 105.11 | 31.73 | 11 | 72.62 |
| 2.88 | 26.4 | 30.95 | 6.4 | 40.82 | ||||||
| 2 | 57 | 75 | P. mirabilis | piperacillin | CVVHD | 10.04 | 241.85 | - | 12.1 | 118.62 |
| 5.2 | 297.15 | 32.45 | 11.6 | 111.15 | ||||||
| 3 | 77 | 91 | E.coli, C. albicans | - | diuresis supported with FSD | 2.09 | 231.29 | 28.33 | 4.8 | 66.99 |
| 1.21 | 140.11 | - | 5.1 | 58.17 | ||||||
| 1.1 | 124.79 | 30.78 | 6.9 | 63.08 | ||||||
| 4 | 59 | 84 | S. marcescens, S. aureus | - | - | 30.2 | 78.93 | 23.4 | 18.3 | 109.19 |
| 5 | 71 | 78 | S. aureus | ceftriaxone | CVVHD | 4.72 | 369.99 | 27.64 | 11.1 | 220.28 |
| 6.24 | 366.4 | 34.44 | 16 | 196.23 | ||||||
| 6 | 92 | 68 | K. pneumoniae | piperacillin | diuresis supported with FSD | 2.64 | 377.4 | - | 23.3 | 198.76 |
| 2.47 | 324.75 | 21.52 | 27 | 197.61 | ||||||
| 7 | 30 | 78 | Candida spp. | Piperacillin | diuresis supported with FSD | 2.36 | 327.57 | 39.73 | 14.4 | 178.12 |
| Pat. # | Age [years] | Body Weight [kg] | Microorganisms Found | Other Drugs | Renal Impairment Therapy | PCT [ng/mL] | CRP [mg/L] | Albumins [g/L] | Urea [mmol/L] | Creatinine [µmol/L] |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients receiving PIPERACILLIN | ||||||||||
| 1 | 66 | 86 | P. mirabilis, E. faecalis | - | CVVHD | 0.71 | 263 | 31.57 | 16 | 164 |
| 2 | 28 | 76 | P. aeruginosa, S. marcescens | - | - | 0.13 | 35.66 | 33.1 | 11.6 | 29.36 |
| 3 | 68 | 84 | C. freundi | - | - | 37.96 | 61 | 22.56 | 9.1 | 117 |
| 4 | 69 | 78 | E. coli, Candida sp. | - | - | 0.06 | 60.14 | 31.64 | 4 | 28.72 |
| 5 | 46 | 67 | K. pneumoniae | - | - | 0.28 | 184.35 | 32.86 | 10.1 | 53.53 |
| 6 | 77 | 79 | C. albicans | - | - | 2.37 | 195.97 | 28.58 | 13.6 | 105.33 |
| Patients receiving IMIPENEM | ||||||||||
| 1 | 71 | 78 | S. aureus | ceftriaxone | CVVHD | 4.72 | 369.99 | 27.64 | 11.1 | 220.28 |
| 6.24 | 366.4 | 34.44 | 16 | 196.23 | ||||||
| 2 | 92 | 68 | K. pneumoniae | piperacillin | diuresis supported with FSD | 2.64 | 377.4 | - | 23.3 | 198.76 |
| 2.47 | 324.75 | 21.52 | 27 | 197.61 | ||||||
| 3 | 30 | 78 | Candida spp. | piperacillin | diuresis supported with FSD | 2.36 | 327.57 | 39.73 | 14.4 | 178.12 |
| Pat. # | Sample Number | Antibiotic Treatment before Sampling [days] | Concentration in Plasma before Infusion [mg/L] | Concentration in Plasma after Infusion [mg/L] |
|---|---|---|---|---|
| Patients receiving PIPERACILLIN | ||||
| 1 | PIP-1 | 1 | 162.869 | 307.806 |
| 2 | PIP-2 | 7 | 115.500 | 286.148 |
| 3 | PIP-3 | 3 | 61.603 | 133.925 |
| PIP-4 | 4 | 58.241 | 138.191 | |
| PIP-5 | 5 | 44.869 | 203.747 | |
| 4 | PIP-6 | 6 | 54.496 | 278.231 |
| PIP-7 | 7 | 62.556 | 198.964 | |
| 5 | PIP-8 | 1 | 215.263 | 461.537 |
| 6 | PIP-9 | 1 | 166.428 | 370.821 |
| 7 | PIP-10 | 3 | 92.074 | 189.133 |
| 8 | PIP-11 | 0 | 14.533 | 109.166 |
| 9 | PIP-12 | 2 | 53.270 | 130.710 |
| 10 | PIP-13 | 1 | 22.617 | 98.457 |
| 11 | PIP-14 | 2 | 183.280 | 307.027 |
| Patients receiving IMIPENEM | ||||
| 1 | IMI-1 | 2 | 0.918 | 15.344 |
| IMI-2 | 4 | 1.646 | 16.283 | |
| 2 | IMI-3 | 4 | 0.756 | 8.176 |
| IMI-4 | 5 | 0.293 | 17.185 | |
| 3 | IMI-5 | 4 | 1.650 | 18.394 |
| IMI-6 | 5 | 0.835 | 5.627 | |
| IMI-7 | 6 | 1.157 | 12.919 | |
| 4 | IMI-8 | n.a. | 15.034 | 38.816 |
| 5 | IMI-9 | 0 | 7.001 | 25.113 |
| IMI-10 | 3 | 2.958 | 10.219 | |
| 6 | IMI-11 | 1 | 5.067 | 13.399 |
| IMI-12 | 2 | 5.291 | 13.164 | |
| 7 | IMI-13 | 0 | 0.707 | 5.852 |
| Pat. # | Sample Number | Concentration after Infusion in PLASMA [mg/L] | Concentration after Infusion in BAL [mg/L] | BAL/Plasma % |
|---|---|---|---|---|
| Patients receiving PIPERACILLIN | ||||
| 1 | PIP-I | 201.687 | 3.794 | 1.88 |
| 2 | PIP-II | 263.928 | 6.271 | 2.38 |
| 3 | PIP-III | 309.428 | 6.995 | 2.26 |
| 4 | PIP-IV | 109.166 | 2.236 | 2.05 |
| 5 | PIP-V | 98.457 | 2.673 | 2.71 |
| 6 | PIP-VI | 307.027 | 7.711 | 2.51 |
| Patients receiving IMIPENEM | ||||
| 1 | IMI-I | 25.113 | 2.801 | 11.15 |
| 2 | IMI-II | 10.219 | 0.576 | 5.64 |
| IMI-III | 13.399 | 0.298 | 2.22 | |
| 3 | IMI-IV | 13.164 | 1.189 | 9.03 |
| IMI-V | 5.852 | 0.087 | 1.49 | |
| Type of Sorbent | Number of Layers | Oven Temperature (°C) | Curing Time (min) |
|---|---|---|---|
| C18 | 10 | 180 °C | 2 min |
| DVB | 8 | 110 °C | 3 min |
| PCA | 10 | 110 °C | 3 min |
| SCX | 10 | 110 °C | 3 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarski, R.; Żuchowska, K.; Filipiak, W. Quantitative Determination of Unbound Piperacillin and Imipenem in Biological Material from Critically Ill Using Thin-Film Microextraction-Liquid Chromatography-Mass Spectrometry. Molecules 2022, 27, 926. https://doi.org/10.3390/molecules27030926
Włodarski R, Żuchowska K, Filipiak W. Quantitative Determination of Unbound Piperacillin and Imipenem in Biological Material from Critically Ill Using Thin-Film Microextraction-Liquid Chromatography-Mass Spectrometry. Molecules. 2022; 27(3):926. https://doi.org/10.3390/molecules27030926
Chicago/Turabian StyleWłodarski, Robert, Karolina Żuchowska, and Wojciech Filipiak. 2022. "Quantitative Determination of Unbound Piperacillin and Imipenem in Biological Material from Critically Ill Using Thin-Film Microextraction-Liquid Chromatography-Mass Spectrometry" Molecules 27, no. 3: 926. https://doi.org/10.3390/molecules27030926