Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights
Abstract
:1. Introduction
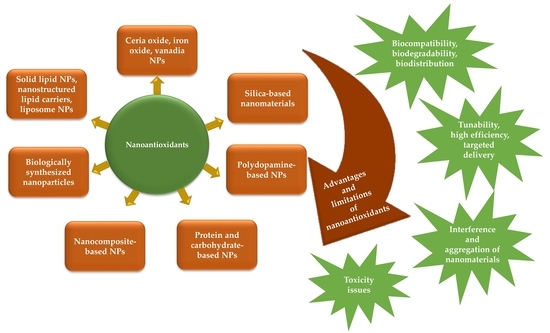
2. Pioneer Types of Nanoantioxidants
2.1. Metal Oxide-Based Nanoantioxidants
2.1.1. Nanoceria or Cerium Oxide-Based Nanoantioxidants
2.1.2. Other Metal Oxide-Based Nanoantioxidants
2.2. Mesoporous Silica or Silicon Dioxide-Based Nanoantioxidants
2.3. Nanocomposite-Based Nanoantioxidants
2.4. PDA-Based Nanoantioxidants
2.5. Polysaccharide-and Protein-Based Nanoantioxidants
2.6. Biologically Fabricated Nanoantioxidants
3. Solid-Lipid-Based NPs, Nanostructured Lipid Carriers, and Liposome NPs as Efficient Nanoencapsulation Systems
3.1. Solid-Lipid-Based NPs and Nanostructured Lipid Carriers
3.2. Liposomes and Their New Generations
4. Toxicity Concerns of Nanoantioxidant Applications
5. Advantages of Nanoantioxidant Applications
6. Limitations of Nanoantioxidant Applications
6.1. Limitations of Nanoantioxidant Methods of Detection and Measurements
6.2. Other Limitations of Nanoantioxidant Applications
- Use of well-dispersed nanomaterials (not-agglomerated);
- Application of washing and separation steps (e.g., centrifugation, filtration, and precipitation);
- Use of appropriate conditions similar to those of biological systems;
- Application of concentrations below toxicity ranges.
7. Future Insights of Nanoantioxidant Applications
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, F.; Gonçalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020, 34, 101475–101491. [Google Scholar] [CrossRef]
- Giam, B.; Kaye, D.M.; Rajapakse, N.W. Role of renal oxidative stress in the pathogenesis of the cardiorenal syndrome. Heart Lung Circ. 2016, 25, 874–880. [Google Scholar] [CrossRef]
- Chen, T.; Luo, W.; Wu, G.; Wu, L.; Huang, S.; Li, J.; Wang, J.; Hu, X.; Huang, W.; Liang, G. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol. Appl. Pharmacol. 2019, 370, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Doridot, L.; Jeljeli, M.; Chêne, C.; Batteux, F. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019, 25, 101122. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Granese, R.; Falsaperla, R.; Reiter, R.J.; Corsello, G.; Gitto, E. Role of oxidative stress in neonatal respiratory distress syndrome. Free Radic. Biol. Med. 2019, 142, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Hu, R.-C.; Li, J.; Chen, B.-B.; Dai, A.-G. α1-Antitrypsin alleviates inflammation and oxidative stress by suppressing autophagy in asthma. Cytokine 2021, 141, 155454. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, L.; Liang, Y.; Peng, D.; Aschner, M.; Jiang, Y. Signal transduction associated with lead-induced neurological disorders: A Review. Food Chem. Toxicol. 2021, 150, 112063. [Google Scholar] [CrossRef]
- Dastmalchi, N.; Baradaran, B.; Latifi-Navid, S.; Safaralizadeh, R.; Khojasteh, S.M.B.; Amini, M.; Roshani, E.; Lotfinejad, P. Antioxidants with two faces toward cancer. Life Sci. 2020, 258, 118186. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, J.; Wang, H. MiR-30a-3p ameliorates oxidative stress in rheumatoid arthritis synovial fibroblasts via activation of Nrf2-ARE signaling pathway. Immunol. Lett. 2021, 232, 1–8. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Introductory remarks. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985. [Google Scholar]
- Harman, D. Free radical theory of aging suggests that free radicals produced during normal metabolic process later react with and damage important molecules. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flieger, J.; Flieger, W.; Baj, J. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; Granville, D.; Meyer, T.J. Proton-coupled electron transfer. Chem Rev. 2012, 107, 5004–5064. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanism of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543–7754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Qiang, M.; Li, F.; Zhang, H.; Zhang, S. Theoretical investigation on the antioxidative activity of anthocyanidins: A DFT/B3LYP study. Dyes Pigment. 2014, 103, 175–182. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of antioxidant properties of agents from the perspective of their action mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Marchi, R.C.; Campos, I.A.S.; Santana, V.T.; Carlos, R.M. Chemical implications and considerations on techniques used to assess the in vitro antioxidant activity of coordination compounds. Coord. Chem. Rev. 2022, 451, 214275. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational study of ortho-substituent effects on antioxidant activities of phenolic dendritic antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstractions. 1. The reactions of phenols with 2, 2-diphenyl-1-picrylhydrazyl (dpph•) in alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [Green Version]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 3. Novel kinetics in sequential proton loss electron transfer chemistry. J. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Diduk, R.; Galano, A.; Tan, D.X.; Reiter, R.J. The key role of the sequential proton loss electron transfer mechanism on the free radical scavenging activity of some melatonin-related compounds. Theor. Chem. Acc. 2016, 135, 38–43. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef] [Green Version]
- Amić, D.; Stepanić, V.; Lučić, B.; Marković, Z.; Dimitrić Marković, J.M. PM6 study of free radical scavenging mechanisms of flavonoids: Why does O-H bond dissociation enthalpy effectively represent free radical scavenging activity? J. Mol. Model. 2013, 19, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Fifen, J.J.; Dhaouadi, Z.; Nsangou, M.; Holtomo, O.; Jaidane, N. “Proton-coupled electron transfer in the reaction of 3,4-dihydroxyphenylpyruvic acid with reactive species in various media. Int. J. Chem. Phys. 2015, 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- MacAleese, L.; Hermelin, S.; El Hage, K.; Chouzenoux, P.; Kulesza, A.; Antoine, R.; Bonacina, L.; Meuwly, M.; Wolf, J.P.; Dugourd, P. Sequential proton coupled electron transfer (PCET): Dynamics observed over 8 orders of magnitude in time. J. Am. Chem. Soc. 2016, 138, 4401–4407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Lo Conte, M.; Carroll, K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013, 288, 26480–26488. [Google Scholar] [CrossRef] [Green Version]
- van Lith, R.; Ameer, G.A. Antioxidant polymers as biomaterial. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Elsevier Inc.: San Diego, CA, USA, 2016; pp. 251–296. [Google Scholar]
- Liu, Y.; Shi, J. Antioxidative nanomaterials and biomedical applications. Nano Today 2019, 27, 146–177. [Google Scholar] [CrossRef]
- Azab, A.E.; Adwas, A.A.; Elsayed, A.S.I.; Adwas, A.A.; Elsayed, A.S.I.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Jacewicz, D.; Chmurzyński, L. The impact of environmental contamination on the generation of reactive oxygen and nitrogen species—Consequences for plants and humans. Environ. Int. 2018, 119, 133–151. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial nanoparticle antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; Andreescu, S., Hepel, M., Eds.; Oxford University Press Inc.: New York, NY, USA, 2012; pp. 235–253. [Google Scholar]
- Medhe, S.; Bansal, P.; Srivastava, M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014, 4, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Chatterjee, S. Green synthesis of metal/metal oxide nanoparticles toward biomedical applications: Boon or bane. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier Health Sciences: London, UK, 2019; pp. 265–301. [Google Scholar]
- Huang, K.-J.; Wu, S.-R.; Shieh, D.-B. Zero-valent iron nanoparticles inhibited head and neck cancer cells growth: A pilot evaluation and mechanistic characterization. Free Radic. Biol. Med. 2017, 108, S39. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Samuel, M.S.; Verma, T.N.; Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Pugazhendhi, A.; Duc, P.A. Zinc oxide nanoparticles (ZnONPs)-induced antioxidants and photocatalytic degradation activity from hybrid grape pulp extract (HGPE). Biocatal. Agric. Biotechnol. 2020, 28, 101730–101737. [Google Scholar] [CrossRef]
- Kokalari, I.; Gassino, R.; Giovannozzi, A.M.; Croin, L.; Gazzano, E.; Bergamaschi, E.; Rossi, A.M.; Perrone, G.; Riganti, C.; Ponti, J. Pro-and anti-oxidant properties of near-infrared (NIR) light responsive carbon nanoparticles. Free Radic. Biol. Med. 2019, 134, 165–176. [Google Scholar] [CrossRef]
- Mansour Lakourj, M.; Norouzian, R.S.; Esfandyar, M.; Ghasemi Mir, S. Conducting nanocomposites of polypyrrole-co-polyindole doped with carboxylated CNT: Synthesis approach and anticorrosion/antibacterial/antioxidation property. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 261, 114673–114688. [Google Scholar] [CrossRef]
- Shah, S.T.; Yehya, A.W.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–38. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447–466. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, O.; Zayachkivska, A.; Vaiserman, A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: A hypothetical background and translational perspectives. Oxid. Med. Cell. Longev. 2018, 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftekhari, A.; Dizaj, S.M.; Chodari, L.; Sunar, S.; Hasanzadeh, A.; Ahmadian, E.; Hasanzadeh, M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018, 103, 1018–1027. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and nanotechnology: Promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Healthc. Mater. 2019, 9, 1901589–1901617. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2020, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Halliwell, B. How to characterize a biological antioxidant. Free Radic. Res. Commun. 1990, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Galano, A. Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry-based protocols. J. Mex. Chem. Soc. 2017, 59, 231–262. [Google Scholar] [CrossRef] [Green Version]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omran, B.A. Fundamentals of Nanotechnology and Nanobiotechnology. In Nanobiotechnology: A Multidisciplinary Field of Science; Springer: Cham, Switzerland, 2020; pp. 1–36. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20–49. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Xu, Y.; Musumeci, V.; Aymonier, C. Chemistry in supercritical fluids for the synthesis of metal nanomaterials. React. Chem. Eng. 2019, 4, 2030–2054. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902–915. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.Y.; Song, S.Y.; Go, S.; Sohn, H.S.; Baik, S.; Soh, M.; Kim, K.; Kim, D.; Kim, H.-C. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 2019, 13, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.F.; Ameer, G.A. A cooperative copper metal–organic framework-hydrogel system improves wound healing in diabetes. Adv. Funct. Mater. 2016, 27, 1604872–1604882. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–based silica particles as flavonoid carrier for drug delivery applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [Green Version]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003–42026. [Google Scholar] [CrossRef]
- Moskvin, M.; Huntošová, V.; Herynek, V.; Matouš, P.; Michalcová, A.; Lobaz, V.; Zasońska, B.; Šlouf, M.; Seliga, R.; Horák, D. In vitro cellular activity of maghemite/cerium oxide magnetic nanoparticles with antioxidant properties. Colloids Surf. B Biointerfaces 2021, 204, 111824–111833. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 32004–32033. [Google Scholar] [CrossRef]
- Chandar, N.K.; Jayavel, R. Synthesis and characterization of C14TAB passivated cerium oxide nanoparticles prepared by co-precipitation route. Phys. E Low-Dimens. Syst. Nanostructures 2014, 58, 48–51. [Google Scholar] [CrossRef]
- Derakhshandeh, P.G.; Soleimannejad, J. Sonochemical synthesis of a new nano-sized cerium (III) supramolecular compound; Precursor for nanoceria. Ultrason. Sonochem. 2016, 31, 122–128. [Google Scholar] [CrossRef]
- Akbari, A.; Khammar, M.; Taherzadeh, D.; Rajabian, A.; Zak, A.K.; Darroudi, M. Zinc-doped cerium oxide nanoparticles: Sol-gel synthesis, characterization, and investigation of their in vitro cytotoxicity effects. J. Mol. Struct. 2017, 1149, 771–776. [Google Scholar] [CrossRef]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant potential and toxicity study of the cerium oxide nanoparticles synthesized by microwave-mediated synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Khadar, Y.A.S.; Balamurugan, A.; Devarajan, V.P.; Subramanian, R.; Kumar, S.D. Synthesis, characterization and antibacterial activity of cobalt doped cerium oxide (CeO2: Co) nanoparticles by using hydrothermal method. J. Mater. Res. Technol. 2019, 8, 267–274. [Google Scholar] [CrossRef]
- Mishra, S.; Priyadarshinee, M.; Debnath, A.K.; Muthe, K.P.; Mallick, B.C.; Das, N.; Parhi, P. Rapid microwave assisted hydrothermal synthesis cerium vanadate nanoparticle and its photocatalytic and antibacterial studies. J. Phys. Chem. Solids 2020, 137, 109211–109220. [Google Scholar] [CrossRef]
- Sundararajan, V.; Venkatasubbu, G.D.; Sheik Mohideen, S. Investigation of therapeutic potential of cerium oxide nanoparticles in Alzheimer’s disease using transgenic Drosophila. 3 Biotech 2021, 11, 159–170. [Google Scholar] [CrossRef]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Källman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017, 7, 1–20. [Google Scholar] [CrossRef]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [Green Version]
- Zarkovic, N. Roles and functions of ROS and RNS in cellular physiology and pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and detrimental effects of reactive oxygen species on lifespan: A comprehensive review of comparative and experimental studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nano Biotechnol. 2019, 17, 84. [Google Scholar] [CrossRef]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Fresnais, J.; Muller, P.; Theodoly, O.; Berret, J.-F.; Chapel, J.-P. Interfacial activity of phosphonated-PEG functionalized cerium oxide nanoparticles. Langmuir 2012, 28, 11448–11456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Perez, J.M.; Webster, T.J. Inhibited growth of Pseudomonas aeruginosa by dextran-and polyacrylic acid-coated ceria nanoparticles. Int. J. Nanomed. 2013, 8, 3395–3399. [Google Scholar]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Hoseini, S.J.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium oxide nanoparticles (Nanoceria): Hopes in soft tissue engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, B.H. Antioxidant activity of levan coated cerium oxide nanoparticles. Carbohydr. Polym. 2016, 150, 400–407. [Google Scholar] [CrossRef]
- Weaver, J.D.; Stabler, C.L. Antioxidant cerium oxide nanoparticle hydrogels for cellular encapsulation. Acta Biomater. 2015, 16, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, Z.; Charbgoo, F.; Darroudi, M. Impact of physicochemical properties of cerium oxide nanoparticles on their toxicity effects. Ceram. Int. 2017, 43, 14572–14581. [Google Scholar] [CrossRef]
- Fa, M.; Yang, D.; Gao, L.; Zhao, R.; Luo, Y.; Yao, X. The effect of AuNP modification on the antioxidant activity of CeO2 nanomaterials with different morphologies. Appl. Surf. Sci. 2018, 457, 352–359. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Ragg, R.; Natalio, F.; Tahir, M.N.; Janssen, H.; Kashyap, A.; Strand, D.; Strand, S.; Tremel, W. Molybdenum trioxide nanoparticles with intrinsic sulfite oxidase activity. ACS Nano 2014, 8, 5182–5189. [Google Scholar] [CrossRef]
- Olvera Salazar, A.; García Hernández, M.; López Camacho, P.Y.; López Marure, A.; Reyes de la Torre, A.I.; de Jesús Morales Ramírez, A.; Hernández Santiago, F.; Aguilera Vázquez, L. Influence of Eu3+ doping content on antioxidant properties of Lu2O3 sol-gel derived nanoparticles. Mater. Sci. Eng. C 2016, 69, 850–855. [Google Scholar] [CrossRef]
- Javed, R.; Ahmed, M.; ul Haq, I.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Tabassum, S.; Zia, M. Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Juarez-Moreno, K.; Ayala, M.; Vazquez-Duhalt, R. Antioxidant capacity of poly (ethylene glycol) (PEG) as protection mechanism against hydrogen peroxide inactivation of peroxidases. Appl. Biochem. Biotechnol. 2015, 177, 1364–1373. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella Jr, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef] [PubMed]
- Bakhshian Nik, A.; Zare, H.; Razavi, S.; Mohammadi, H.; Torab Ahmadi, P.; Yazdani, N.; Bayandori, M.; Rabiee, N.; Izadi Mobarakeh, J. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115–110129. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634–1902647. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Gimenez, C.; de la Torre, C.; Gorbe, M.; Aznar, E.; Sancenon, F.; Murguía, J.R.; Martinez-Manez, R.; Marcos, M.D.; Amoros, P. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 2017, 13, 1602225–1602236. [Google Scholar] [CrossRef]
- Zhang, R.; Hua, M.; Liu, H.; Li, J. How to design nanoporous silica nanoparticles in regulating drug delivery: Surface modification and porous control. Mater. Sci. Eng. B 2021, 263, 114835–114849. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly (tannic acid)-silica nanoparticle composites with antioxidant properties. Microporous Mesoporous Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Peralta, I.; Alonso, M.R.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf. B Biointerfaces 2018, 169, 82–91. [Google Scholar] [CrossRef]
- Das, S.; Batuta, S.; Alam, M.N.; Fouzder, C.; Kundu, R.; Mandal, D.; Begum, N.A. Antioxidant flavone analog functionalized fluorescent silica nanoparticles: Synthesis and exploration of their possible use as biomolecule sensor. Colloids Surf. B Biointerfaces 2017, 157, 286–296. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, S.J.; Jeong, S.W.; Kim, H.C.; Park, G.Y.; Lee, S.G.; Choi, J.H. Antioxidative and anti-inflammatory activities of quercetin-loaded silica nanoparticles. Colloids Surf. B Biointerfaces 2016, 143, 511–517. [Google Scholar] [CrossRef]
- Kumar, S.; Sarita; Nehra, M.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog. Polym. Sci. 2018, 80, 1–38. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Yan, L.; Gonca, S.; Zhu, G.; Zhang, W.; Chen, X. Layered double hydroxide nanostructures and nanocomposites for biomedical applications. J. Mater. Chem. B 2019, 7, 5583–5601. [Google Scholar] [CrossRef] [Green Version]
- Safdari, M.; Al-Haik, M.S. Synergistic electrical and thermal transport properties of hybrid polymeric nanocomposites based on carbon nanotubes and graphite nanoplatelets. Carbon 2013, 64, 111–121. [Google Scholar] [CrossRef]
- Lee, R.-J.; Tamm, T.; Temmer, R.; Aabloo, A.; Kiefer, R. Two formation mechanisms and renewable antioxidant properties of suspensible chitosan–PPy and chitosan–PPy–BTDA composites. Synth. Met. 2013, 164, 6–11. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Liu, C.; Ju, E.; Zhang, Y.; Ren, J.; Qu, X. Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. 2016, 128, 6758–6762. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Liang, Y.; Lin, Y.; Liu, Z.; Niu, H.; Xu, Y. Establishment of anti-oxidation platform based on few-layer molybdenum disulfide nanosheet-coated titanium dioxide nanobelt nanocomposite. J. Colloid Interface Sci. 2021, 601, 167–176. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Monitoring the intracellular distribution and ROS scavenging potential of carbon dot–cerium oxide nanocomposites in fibroblast cells. ChemNanoMat 2016, 2, 226–235. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Hao, L.; Jiang, W.; Gong, X.; Hu, Y.; Chen, Z. A facile method to fabricate carbon-encapsulated Fe3O4 core/shell composites. Nanotechnology 2007, 18, 35602–35608. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, D.; Ranjithkumar, R.; Vasantharaj, S.; Chandarshekar, B.; Bhuvaneshwari, V. Synthesis and characterization of chitosan/iron oxide nanocomposite for biomedical applications. Int. J. Biol. Macromol. 2019, 132, 880–887. [Google Scholar] [CrossRef]
- Rajeswari, R.; Gurumallesh Prabu, H. Palladium—Decorated reduced graphene oxide/zinc oxide nanocomposite for enhanced antimicrobial, antioxidant and cytotoxicity activities. Process. Biochem. 2020, 93, 36–47. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials synthesis through microfluidic methods: An updated overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Cui, X.; Yin, Y.; Ma, Z.; Yin, Y.; Guan, Y.; Rong, S.; Gao, J.; Niu, Y.; Li, M. Polydopamine used as hollow capsule and core–shell structures for multiple applications. Nano 2015, 10, 1530003–1530026. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Fan, H.; Wang, L.; Jin, Z. Oxidative self-polymerization of dopamine in an acidic environment. Langmuir 2015, 31, 11671–11677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, Y.; Duan, Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control. Release 2018, 290, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, Y.; Melnikov, P.; Baklaushev, V.; Gabashvili, A.; Osipova, N.; Mantrov, S.; Ermolenko, Y.; Maksimenko, O.; Gorshkova, M.; Balabanyan, V. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017, 524, 77–90. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598–117608. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E.; et al. Polydopamine nanoparticles as an organic and biodegradable multitasking tool for neuroprotection and remote neuronal stimulation. ACS Appl. Mater. Interfaces 2020, 12, 35782–35798. [Google Scholar] [CrossRef]
- Hemmati, S.; Zangeneh, M.M.; Zangeneh, A. CuCl2 anchored on polydopamine coated-magnetic nanoparticles (Fe3O4@PDA/Cu (II)): Preparation, characterization and evaluation of its cytotoxicity, antioxidant, antibacterial, and antifungal properties. Polyhedron 2020, 177, 114327–114336. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Liu, Z.; Wang, Y.; Lin, D.; Chen, L.; Dai, J.; Lin, K.; Shen, S.G. Polydopamine nanoparticles as dual-task platform for osteoarthritis therapy: A scavenger for reactive oxygen species and regulator for cellular powerhouses. Chem. Eng. J. 2021, 417, 129284–129300. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives—Formation Principles, characterization techniques, and biomedical applications. Macromol. Biosci. 2020, 20, 1900415–1900454. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Vangelie, E.; Campos, R.; Rodriguez, P.; Susana, L.; Torres, A.; Armando, L.; Torres, D.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71–104. [Google Scholar] [CrossRef] [Green Version]
- Matos, B.N.; Reis, T.A.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Palacios, A.; Ahmed, A.; Lane, M.A.; Aminabhavi, T.M. Targeted delivery of small interfering RNA to colon cancer cells using chitosan and PEGylated chitosan nanoparticles. Carbohydr. Polym. 2016, 147, 323–332. [Google Scholar] [CrossRef]
- Cevher, E.; Salomon, S.K.; Somavarapu, S.; Brocchini, S.; Alpar, H.O. Development of chitosan-pullulan composite nanoparticles for nasal delivery of vaccines: In vivo studies. J. Microencapsul. 2015, 32, 769–783. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Mandal, S.; Khandalavala, K.; Pham, R.; Bruck, P.; Varghese, M.; Kochvar, A.; Monaco, A.; Prathipati, P.K.; Destache, C.; Shibata, A. Cellulose acetate phthalate and antiretroviral nanoparticle fabrications for HIV pre-exposure prophylaxis. Polymers 2017, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenbach, B.B.; Schmid, I.; Wich, P.R. Amphiphilic polysaccharide block copolymers for pH-responsive micellar nanoparticles. Biomacromolecules 2017, 18, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Sauraj; Kumar, S.U.; Kumar, V.; Priyadarshi, V.K.R.; Gopinath, P.; Negi, Y.S. pH-responsive prodrug nanoparticles based on xylan-curcumin conjugate for the efficient delivery of curcumin in cancer therapy. Carbohydr. Polym. 2018, 188, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides-based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067–100091. [Google Scholar] [CrossRef]
- Muhammad, G.; Hussain, M.A.; Amin, M.; Hussain, S.Z.; Hussain, I.; Bukhari, S.N.A.; Naeem-ul-Hassan, M. Glucuronoxylan-mediated silver nanoparticles: Green synthesis, antimicrobial and wound healing applications. RSC Adv. 2017, 7, 42900–42908. [Google Scholar] [CrossRef] [Green Version]
- Ganie, S.A.; Rather, L.J.; Li, Q. A review on anticancer applications of pullulan and pullulan derivative nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100115–100132. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459–104473. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Li, K.; Xia, C.; Li, J. Sodium alginate-assisted route to antimicrobial biopolymer film combined with aminoclay for enhanced mechanical behaviors. Ind. Crop. Prod. 2019, 135, 271–282. [Google Scholar] [CrossRef]
- John, T.; Nwude, F.; Singh, S.; Odunayo, O.; Benjakul, S.; Rujiralai, T. Synthesis of gold nanoparticles/polyaniline boronic acid/sodium alginate aqueous nanocomposite based on chemical oxidative polymerization for biological applications. Int. J. Biol. Macromol. 2021, 179, 196–205. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed. Res. Int. 2014, 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Sandra, F.; Khaliq, N.U.; Sunna, A.; Care, A. Developing protein-based nanoparticles as versatile delivery systems for cancer therapy and imaging. Nanomaterials 2019, 9, 1329. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.; Guimarães, K.L.; Cerize, N.; Tunussi, A.S.; Poço, J.G. Nano spray drying as an innovative technology for encapsulating hydrophilic active pharmaceutical ingredients (API). J. Nanomed. Nanotechnol 2013, 4, 1000186–1000192. [Google Scholar] [CrossRef] [Green Version]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of cerium oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Whitehead, K.A.; Baek, K.-H. One-pot bioinspired synthesis of fluorescent metal chalcogenide and carbon quantum dots: Applications and potential biotoxicity. Colloids Surf. B Biointerfaces 2021, 200, 111578–111597. [Google Scholar] [CrossRef] [PubMed]
- De Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Anastas, P.T. Green chemistry and the role of analytical methodology development. Crit. Rev. Anal. Chem. 1999, 29, 167–175. [Google Scholar] [CrossRef]
- O’Neil, N.J.; Scott, S.; Relph, R.; Ponnusamy, E. Approaches to incorporating green chemistry and safety into laboratory culture. J. Chem. Educ. 2020, 98, 84–91. [Google Scholar] [CrossRef]
- Omran, B.A. Biosynthesized nanomaterials via processing of different plant parts (phytonanotechnology) and biovalorization of agro-industrial wastes to nano-sized valuable products. In Nanobiotechnology: A Multidisciplinary Field of Science; Springer: Cham, Switzerland, 2020; pp. 145–184. [Google Scholar]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and antibacterial potential of silver nanoparticles: Biogenic synthesis utilizing apple extract. J. Pharm. 2016, 2016, 8. [Google Scholar] [CrossRef]
- Fafal, T.; Taştan, P.; Tüzün, B.S.; Ozyazici, M.; Kivcak, B. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Asphodelus aestivus Brot. aerial part extract. S. Afr. J. Bot. 2017, 112, 346–353. [Google Scholar] [CrossRef]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.; Rai, V.R.; Ramachandra, Y.L. Results in Physics anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019, 1, 498–514. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Saini, H.; Kumar, D.; Pasi, S.; Agrawal, V. Bioengineering of Piper longum L. extract mediated silver nanoparticles and their potential biomedical applications. Mater. Sci. Eng. C 2019, 104, 109984–110001. [Google Scholar] [CrossRef]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Srivastava, M.M. Single-step green route synthesis of Au/Ag bimetallic nanoparticles using clove buds extract: Enhancement in antioxidant bio-efficacy and catalytic activity. Mater. Sci. Eng. C 2020, 116, 111153–111166. [Google Scholar] [CrossRef] [PubMed]
- Yarrappagaari, S.; Gutha, R.; Narayanaswamy, L.; Thopireddy, L.; Benne, L.; Mohiyuddin, S.S.; Vijayakumar, V.; Saddala, R.R. Eco-friendly synthesis of silver nanoparticles from the whole plant of Cleome viscosa and evaluation of their characterization, antibacterial, antioxidant and antidiabetic properties. Saudi J. Biol. Sci. 2020, 27, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheshwari Nallal, V.; Prabha, K.; VethaPotheher, I.; Ravindran, B.; Baazeem, A.; Woong Chang, S.; Aderonke Otunola, G.; Razia, M. Sunlight-driven rapid and facile synthesis of silver nanoparticles using Allium ampeloprasum extract with enhanced antioxidant and antifungal activity. Saudi J. Biol. Sci. 2021, 28, 3660–3668. [Google Scholar] [CrossRef]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. C 2020, 107, 110207–110218. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Zhang, H.; Ashraf, M.; Fang, H.; Zeng, X.; Wu, Y.; Khurshid, M.; Zhao, L.; He, Z. Antibacterial and antioxidant activity of exopolysaccharide mediated silver nanoparticle synthesized by Lactobacillus brevis isolated from Chinese koumiss. Colloids Surf. B Biointerfaces 2020, 186, 110734–110745. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agent. Mater. Sci. Eng. C 2020, 112, 110901–110908. [Google Scholar] [CrossRef]
- Kong, Y.; Ahmad, B.; Al-sadoon, M.K.; Fahad, M. Novel green synthesis, chemical characterization, toxicity, colorectal carcinoma, antioxidant, anti-diabetic, and anticholinergic properties of silver nanoparticles: A chemopharmacological study. Arab. J. Chem. 2021, 14, 103193–103203. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Tahar, L.B.; Harrath, A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020, 13, 3112–3122. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Priya Velammal, S.; Devi, T.A.; Amaladhas, T.P. Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticles synthesized using Plumbago zeylanica bark. J. Nanostruct. Chem. 2016, 6, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Selvi, A.M.; Palanisamy, S.; Jeyanthi, S.; Vinosha, M.; Mohandoss, S.; Tabarsa, M.; You, S.G.; Kannapiran, E.; Prabhu, N.M. Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: Antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process. Biochem. 2020, 98, 21–33. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Ghaneialvar, H.; Akbaribazm, M.; Ghanimatdan, M.; Abbasi, N.; Goorani, S.; Pirabbasi, E.; Zangeneh, A. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem. Photobiol. B Biol. 2019, 197, 111556–111569. [Google Scholar] [CrossRef]
- Venugopalan, R.; Pitchai, S.; Devarayan, K.; Swaminathan, V.C. Biogenic synthesis of copper nanoparticles using Borreria hispida (Linn.) extract and its antioxidant activity. Mater. Today Proc. 2020, 33, 4023–4025. [Google Scholar] [CrossRef]
- Merugu, R.; Gothalwal, R.; Kaushik Deshpande, P.; De Mandal, S.; Padala, G.; Chitturi, K.L. Synthesis of Ag/Cu and Cu/Zn bimetallic nanoparticles using toddy palm: Investigations of their antitumor, antioxidant and antibacterial activities. Mater. Today Proc. 2020, 99–105. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Oladipo, A.O.; Msagati, T.A.M.; Lebelo, S.L.; Meddows-Taylor, S.; More, G.K. Novel silver-platinum bimetallic nanoalloy synthesized from Vernonia mespilifolia extract: Antioxidant, antimicrobial, and cytotoxic activities. Arab. J. Chem. 2020, 13, 6639–6648. [Google Scholar] [CrossRef]
- Velsankar, K.; Sudhahar, S.; Maheshwaran, G.; Krishna Kumar, M. Effect of biosynthesis of ZnO nanoparticles via Cucurbita seed extract on Culex tritaeniorhynchus mosquito larvae with its biological applications. J. Photochem. Photobiol. B Biol. 2019, 200, 111650. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sangeetha, E.; Saraswathi, H.; Soundarya, S.; Kumar, N.M. Green fabrication, characterization of Pisonia alba leaf extract derived MgO nanoparticles and its biological applications. Nano-Struct. Nano-Objects 2019, 20, 100380–100385. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Loganathan, S.; Shivakumar, M.S.; Karthi, S.; Nathan, S.S.; Selvam, K. Metal oxide nanoparticle synthesis (ZnO-NPs) of Knoxia sumatrensis (Retz.) DC. Aqueous leaf extract and It’s evaluation of their antioxidant, anti-proliferative and larvicidal activities. Toxicol. Rep. 2021, 8, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.A.; Saurabh Jha, S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Hari Babu, V.V. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Akinola, P.O.; Lateef, A.; Asafa, T.B.; Beukes, L.S.; Hakeem, A.S.; Irshad, H.M. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: Antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon 2020, 6, e04610. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Wang, M.H. Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceram. Int. 2021, 47, 8618–8626. [Google Scholar] [CrossRef]
- Bordoni, M.; Scarian, E.; Rey, F.; Gagliardi, S.; Carelli, S.; Pansarasa, O.; Cereda, C. Biomaterials in neurodegenerative disorders: A promising therapeutic approach. Int. J. Mol. Sci. 2020, 21, 3243. [Google Scholar] [CrossRef]
- Dutta, D.; Mukherjee, R.; Patra, M.; Banik, M.; Dasgupta, R.; Mukherjee, M.; Basu, T. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids Surf. B Biointerfaces 2016, 147, 45–53. [Google Scholar] [CrossRef]
- Karmous, I.; Pandey, A.; Haj, K.B.; Chaoui, A. Efficiency of the green synthesized nanoparticles as new tools in cancer therapy: Insights on plant-based bioengineered nanoparticles, biophysical properties, and anticancer roles. Biol. Trace Elem. Res. 2020, 196, 330–342. [Google Scholar] [CrossRef]
- Sharma, D.; Shandilya, P.; Saini, N.K.; Singh, P.; Thakur, V.K.; Saini, R.V.; Mittal, D.; Chandan, G.; Saini, V.; Saini, A.K. Insights into the synthesis and mechanism of green synthesized antimicrobial nanoparticles, answer to the multidrug resistance. Mater. Today Chem. 2021, 19, 100391. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Pink, D.L.; Loruthai, O.; Ziolek, R.M.; Wasutrasawat, P.; Terry, A.E.; Lawrence, M.J.; Lorenz, C.D. On the structure of solid lipid nanoparticles. Small 2019, 15, 1903156. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- D’Souza, A.; Shegokar, R. Nanostructured lipid carriers (NLCs) for drug delivery: Role of liquid lipid (oil). Curr. Drug Deliv. 2021, 18, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.R.; Shegokar, R.; Keck, M.C. 20 years of lipid nanoparticles (SLN & NLC): Present state of development & industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloid Interface Sci. Commun. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Venegas, J.B.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonia, N.; Narang, J.K.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: Systematic development, characterization and pharmacokinetic evaluation. Colloids Surf. B Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In vitro antioxidant activity and in vivo topical efficacy of lipid nanoparticles co-loadingm idebenone and tocopheryl acetate. Appl. Sci. 2019, 9, 845. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.M.; Das, S.; Chow, P.S.; Macbeath, C. Encapsulation of ferulic acid in lipid nanoparticles as antioxidant for skin: Mechanistic understanding through experiment and molecular simulation. ACS Appl. Nano Mater. 2020, 3, 5351–5361. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing bioavailability and stability of curcumin using solid lipid nanoparticles (CLEN): A covenant for its effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Simão, A.M.S.; Bolean, M.; Cury, T.A.C.; Stabeli, R.G.; Itri, R.; Ciancaglini, P. Liposomal systems as carriers for bioactive compounds. Biophys. Rev. 2015, 7, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984–105001. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571–120586. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Bao, M.; Xin, C.; Wang, L.; Zhang, H.; Zhao, H.; Wang, Z. Green synthesis of gold nanoparticles coated doxorubicin liposomes using procyanidins for light–controlled drug release. Adv. Powder Technol. 2020, 31, 3640–3649. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface Sci. 2019, 263, 52–67. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Dave, V.; Gupta, A.; Singh, P.; Gupta, C.; Sadhu, V.; Reddy, K.R. Synthesis and characterization of celecoxib loaded PEGylated liposome nanoparticles for biomedical applications. Nano-Struct. Nano-Objects 2019, 18, 100288. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 107727. [Google Scholar] [CrossRef]
- Tahara, K.; Nishio, M.; Takeuchi, H. Evaluation of liposomal behavior in the gastrointestinal tract after oral administration using real-time in vivo imaging. Drug Dev. Ind. Pharm. 2018, 44, 608–614. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes coated with thiolated chitosan as drug carriers of curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98–110. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome-based hydrogel on peripheral nerve regeneration in vitro. Mater. Sci. Eng. C 2020, 109, 110560–110573. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, X.; Fan, X.; Li, H.; Xu, B.; Li, X. Whey protein isolate coated liposomes as novel carrier systems for astaxanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900325–1900335. [Google Scholar] [CrossRef]
- Yamazoe, E.; Fang, J.-Y.; Tahara, K. Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm. 2021, 593, 120148. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708–1325726. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [Green Version]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Pantos, A. Formation of artificial multicompartment vesosome and dendrosome as prospected drug and gene delivery carriers. J. Control. Release 2013, 170, 141–152. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Du, J.; Gu, Z.; Zhao, Y. Reactive Oxygen Species-Regulating Strategies Based on Nanomaterials for Disease Treatment. Adv. Sci. 2021, 8, 2002797–2002831. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.U.; Ali, S.I.; Rather, M.A.; Sheikh, W.M.; Altaf, M.; Singh, H.; Mumtaz, P.T.; Mishra, N.C.; Nazir, S.U.; Bashir, S.M. Organoids: A new approach in toxicity testing of nanotherapeutics. J. Appl. Toxicol. 2021. [Google Scholar] [CrossRef]
- Andrysiak, K.; Stępniewski, J.; Dulak, J. Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research. Pflügers Arch. J. Physiol. 2021, 473, 1061–1085. [Google Scholar] [CrossRef]
- Saydé, T.; El Hamoui, O.; Alies, B.; Gaudin, K.; Lespes, G.; Battu, S. Biomaterials for three-dimensional cell culture: From applications in oncology to nanotechnology. Nanomaterials 2021, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, E.; Haarmann-Stemmann, T.; Kapr, J.; Galanjuk, S.; Hartmann, J.; Mertens, P.R.; Kämpfer, A.A.M.; Schins, R.P.F.; Tigges, J.; Koch, K. Stem cells for next level toxicity testing in the 21st century. Small 2021, 17, 2006252. [Google Scholar] [CrossRef]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Trompeta, A.F.; Tsatsakis, A.; Charitidis, C.A.; Kouretas, D. An integrated approach for assessing the in vitro and in vivo redox-related effects of nanomaterials. Environ. Res. 2021, 197, 111083. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, M.B.; Sadeghnia, H.R.; Pasdar, A.; Ghayour-Mobarhan, M.; Riahi-Zanjani, B.; Hashemzadeh, A.; Zare, M.; Darroudi, M. Antioxidant and toxicity studies of biosynthesized cerium oxide nanoparticles in rats. Int. J. Nanomed. 2019, 14, 2915–2926. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, A.; Rao, P.J.; Selvam, G.; Murthy, P.B.; Reddy, P.N. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol. Lett. 2011, 205, 105–115. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.; Cho, W.-S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009, 189, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Liu, T.-P.; Wu, S.-H.; Chen, Y.-P.; Chou, C.-M.; Chen, C.-T. Biosafety evaluations of well-dispersed mesoporous silica nanoparticles: Towards in vivo-relevant conditions. Nanoscale 2015, 7, 6471–6480. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.; Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard. Mater. 2021, 406, 124626–124646. [Google Scholar] [CrossRef]
- Som, C.; Nowack, B.; Krug, H.F.; Wick, P. Toward the development of decision supporting tools that can be used for safe production and use of nanomaterials. Acc. Chem. Res. 2013, 46, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, S.; Li, Z.; Lin, C.; Zhu, Z.; Sun, D.; Bai, R.; Qian, J.; Gao, X.; Chen, G. Autophagic flux blockage in alveolar epithelial cells is essential in silica nanoparticle-induced pulmonary fibrosis. Cell Death Dis. 2019, 10, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, R.; Liu, X.; Qi, Y.; Abulikemu, A.; Zhao, X.; Duan, H.; Zhou, X.; Guo, C.; Sun, Z. Endoplasmic reticulum stress-dependent oxidative stress mediated vascular injury induced by silica nanoparticles in vivo and in vitro. NanoImpact 2019, 14, 100169. [Google Scholar] [CrossRef]
- Zhang, X.; Luan, J.; Chen, W.; Fan, J.; Nan, Y.; Wang, Y.; Liang, Y.; Meng, G.; Ju, D. Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale 2018, 10, 9141–9152. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhouhua, W.; Jie, Z.; Xinlu, F.; Jinqiang, L.; Yuwen, Q.; Zhiying, H. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int. J. Nanomed. 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, R.; Ho, Y.-S.; Hung, C.H.-L.; Liu, Y.; Huang, C.-X.; Chan, H.-N.; Ho, S.-L.; Lui, S.-Y.; Li, H.-W.; Chang, R.C.-C. Silica nanoparticles induce neurodegeneration-like changes in behavior, neuropathology, and affect synapse through MAPK activation. Part. Fibre Toxicol. 2018, 15, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wei, J.; Duan, J.; Guo, C.; Zhang, J.; Ren, L.; Liu, J.; Li, Y.; Sun, Z.; Zhou, X. Silica nanoparticles exacerbates reproductive toxicity development in high-fat diet-treated Wistar rats. J. Hazard. Mater. 2020, 384, 121361. [Google Scholar] [CrossRef]
- Rahdar, A.; Aliahmad, M.; Hajinezhad, M.R.; Samani, M. Xanthan gum-stabilized nano-ceria: Green chemistry based synthesis, characterization, study of biochemical alterations induced by intraperitoneal doses of nanoparticles in rat. J. Mol. Struct. 2018, 1173, 166–172. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, H.; Wang, J.; Wang, D.; Deng, Z.; Li, T.; He, Y.; Yang, Y.; Zhong, S. A water-soluble selenium-enriched polysaccharide produced by Pleurotus ostreatus: Purification, characterization, antioxidant and antitumor activities in vitro. Int. J. Biol. Macromol. 2021, 168, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017, 11, 240–253. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Chen, S.; Zhang, J.; Lu, W. Antioxidant Nanotherapies for the Treatment of Inflammatory Diseases. Front. Bioeng. Biotechnol. 2020, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.K.; Shin, H.; Gouda, S.; Sahoo, S.; Das, G.; Fraceto, L.F.; Patra, J.K. Current advances in nanocarriers for biomedical research and their applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1053–S1062. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.S.; Borbora, H.; Sutar, A.; Dhar, R.S.; Chatterjee, S. Influence of design parameters on multilayered nanoplasmonic structures in modified Kretschmann-Raether configurations. Plasmonics 2020, 15, 1133–1140. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of reactive oxygen and nitrogen species by electron paramagnetic resonance (EPR) technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Kim, S.-U.; Liu, Y.; Nash, K.M.; Zweier, J.L.; Rockenbauer, A.; Villamena, F.A. Fast reactivity of a cyclic nitrone− calix [4] pyrrole conjugate with superoxide radical anion: Theoretical and experimental studies. J. Am. Chem. Soc. 2010, 132, 17157–17173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulaev, V.; Oliver, D.J. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant. Physiol. 2006, 141, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.G.; Rosen, G.M.; Tseitlin, M.; Symmes, B.; Eaton, S.S.; Eaton, G.R. Use of rapid-scan EPR to improve detection sensitivity for spin-trapped radicals. Biophys. J. 2013, 105, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Hanthorn, J.J.; Haidasz, E.; Gebhardt, P.; Pratt, D.A. A versatile fluorescence approach to kinetic studies of hydrocarbon autoxidations and their inhibition by radical-trapping antioxidants. Chem. Commun. 2012, 48, 10141–10143. [Google Scholar] [CrossRef]
- Hay, K.X.; Waisundara, V.Y.; Timmins, M.; Ou, B.; Pappalardo, K.; McHale, N.; Huang, D. High-throughput quantitation of peroxyl radical scavenging capacity in bulk oils. J. Agric. Food Chem. 2006, 54, 5299–5305. [Google Scholar] [CrossRef]
- Jodko-Piórecka, K.; Litwinienko, G. Antioxidant activity of dopamine and L-DOPA in lipid micelles and their cooperation with an analogue of α-tocopherol. Free Radic. Biol. Med. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Mengele, E.A.; Plashchina, I.G.; Kasaikina, O.T. Kinetics of lecithin oxidation in liposomal aqueous solutions. Colloid J. 2011, 73, 815–821. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Filippenko, V.; Presseau, N.; Scaiano, J.C. Oxidation of copper nanoparticles in water: Mechanistic insights revealed by oxygen uptake and spectroscopic methods. Dalt. Trans. 2013, 42, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Soares, M.E.; Duarte, J.A.; Soares, L.; Maia, S.; Gomes, P.; Pereira, E.; Fraga, S.; Carmo, H.; de Lourdes Bastos, M. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur. J. Pharm. Biopharm. 2012, 80, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tournebize, J.; Sapin-minet, A.; Bartosz, G.; Leroy, P. Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 2013, 116, 753–763. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Gainza, E.; Pedraz, J.L. Towards green nanoscience: From extraction to nanoformulation. Biotechnol. Adv. 2021, 46, 107657. [Google Scholar] [CrossRef]









| Preparation Techniques | Suitable Materials | Advantages | References |
|---|---|---|---|
| Templating | Mesoporous materials | Easy operation, precise control over particle size, shape, and structure, less sensitive to operating conditions | [52] |
| Supercritical fluid | Temperature-sensitive materials | Mild operating conditions | [62] |
| Emulsion/solvent evaporation | Polymeric materials | Rapid, mild operating conditions, economical, no toxic solvents | [54] |
| Solvent displacement (Nanoprecipitation) | Lipophilic, polymeric, and bioactive materials | Facile, rapid, reproducible, formation of polymeric NPs, nanospheres, and nanocapsules | [63] |
| Type of NPs | Biological Extract | Size (nm), Shape | Characterization Techniques | Evaluation Assays | IC50 Value (µg/mL) | Antiradical/Antioxidant Activity (%) | References |
|---|---|---|---|---|---|---|---|
| Monometallic-Based Nanoantioxidants | |||||||
| Ag | Malus domestica | 100, spherical | UV/Vis spectroscopy, SEM, Zeta potential | DPPH | - | 75.16 | [173] |
| Ag | Asphodelus aestivus | 50, spherical | UV/Vis spectroscopy, NTA, XRD, TEM, FTIR | DPPH, ABTS•+, H2O2 | - | 67.54, 79.94, 31.67 | [174] |
| Ag | Lippia nodiflora | 30–60, spherical | UV/Vis spectroscopy, FTIR, XRD, SE-EDX, TEM, Zeta potential | DPPH, O2•−, H2O2, •OH | - | 67, 70, 71.1, 69 | [175] |
| Ag | Memecylon umbellatum | 7–22, spherical | UV/Vis spectroscopy, FTIR, HRTEM, XRD, Zeta potential | DPPH | 53.46 | 81.57 | [176] |
| Ag | Calophyllum tomentosum | 24, spherical | UV/Vis spectroscopy, FTIR, XRD, EDX, SEM | DPPH, H2O2, •NO | - | 90, 83.94, 78.46 | [177] |
| Ag | Morus alba | 12–39, spherical | UV/Vis spectroscopy, FTIR, HRTEM, XRD, FESEM, EDX | ABTS•+, DPPH, O2•−, •NO | 25.929, 97.273, 37.097, 70.992 | 95.08, 47.81, 81.92, 64.04 | [178] |
| Ag | Piper longum | 28.8, spherical | UV/Vis spectroscopy, FESEM, XRD, FTIR, HRTEM | DPPH | - | 78.64 | [179] |
| Ag | Rosa canina | 19.75, spherical | UV/Vis spectroscopy, TEM, XPS, XRD | DPPH | - | 86.4 | [180] |
| Ag | Clove bud | 17.94, spherical | UV/Vis spectroscopy, EDX, XRD, FTIR, Zeta potential, cyclic voltammetry | ABTS•+, •OH | 33.03, 47.37 | - | [181] |
| Ag | Cleome viscosa | 50, Rod/spherical/ triangular | UV/Vis spectroscopy, FTIR, XERD, SEM, TEM | DPPH, ABTS•+ | 20.32, 48.5 | 92.8 77.63 | [182] |
| Ag | Allium ampeloprasum | 35, spherical/quasi spherical/ ellipsoidal/ triangular/hexagonal | UV/Vis spectroscopy, FTIR, XRD, TEM | DPPH, ABTS•+, H2O2, •NO, O2•− | 8.93, 18.31, 11.25, 16.51, 23.22 | - | [183] |
| Ag | Aesculus hippocastanum | 50 ± 5, spherical | UV/Vis spectroscopy, FTIR, XRD, SEM, Zeta potential | DPPH, O2•− | - | 93.48 62.9 | [184] |
| Ag | Lactobacillus brevis | 45, spherical | FTIR, XRD, SEM, TEM, elemental analyzer | DPPH, H2O2, •NO | - | 81.4, 70.1, 75.06 | [185] |
| Ag | Achillea millefolium | 20.77 (spherical), 18.53 (triangular), 14.27 (cubic) | UV/Vis spectroscopy, FTIR, SEM, XRD | DPPH | 7.03 | - | [186] |
| Ag | Cannabis sativa | 11.5, spherical | UV/Vis spectroscopy, FTIR, FESEM, TEM | DPPH | 218 | - | [187] |
| Au | Lotus leguminosae | 37, spherical | UV/Vis, IR, TEM, TGA | DPPH | 30.54 | - | [188] |
| Au | Clove bud | 27.12, hexagonal/ polyhedral | UV/Vis spectroscopy, EDX, XRD, FTIR, Zeta potential, cyclic voltammetry | ABTS•+, •OH | 36.76, 50.32 | - | [181] |
| Au | Curcuma pseudomontana | 20, spherical | UV/Vis spectroscopy, SEM, HRTEM, FTIR | DPPH, H2O2, •NO, reducing power, CUPRAC | - | 85.2, 83.2, 84.5, 87.9, 85.6 | [189] |
| Ag, Au | Plumbago zeylanica | 28.47 (spherical), 16.89 (spherical) | UV/Vis spectroscopy, FTIR, TEM, XRD, EDX | DPPH | 56.98 (Ag), 68.53 (Au) | 78.17, 87.34 | [190] |
| Pt | Tragia involucrata | 10, spherical | UV/Vis spectroscopy, XRD, FTIR, FESEM, HRTEM | DPPH | - | 64 ± 0.43 | [191] |
| Cu | Falcaria vulgaris | 20–25, spherical | UV/Vis spectroscopy, XRD, HRTEM, FESEM, FTIR | DPPH | 190 | - | [192] |
| Cu | Borreria hispida | 121 ± 37, quasi spherical | UV/Vis spectroscopy, XRD, SEM, EDX, FTIR | DPPH | 0.6 | - | [193] |
| Bimetallic-Based Nanoantioxidants | |||||||
| Ag/Cu | Borassus flabellifer | 80, - | UV/Vis spectroscopy, FTIR, SEM | DPPH, •OH, H2O2 | - | 58, 48, 42 | [194] |
| Cu/Zn | Borassus flabellifer | 100, - | UV/Vis spectroscopy, FTIR, SEM | DPPH, •OH, H2O2 | - | 40, 38, 28 | [194] |
| Ag/Pt | Vernonia Mespilifolia | 35.5 ± 0.8, spherical | UV/Vis spectroscopy, TEM, EDX, FTIR | DPPH, ABTS•+, FRAP | 19.5 21.6 44.1 | - | [195] |
| Au/Ag | Clove bud | 16.04, spherical | UV/Vis spectroscopy, EDX, XRD, FTIR, Zeta potential, cyclic voltammetry | •OH, ABTS•+ | 30.59 18.27 | - | [181] |
| Metal Oxide-Based Nanoantioxidants | |||||||
| CuO | Cucurbita sp. | 45–65, rod/ rectangular/ hexagonal | UV/Vis spectroscopy, XRD, SEM, EDX, HRTEM | DPPH | 40.81 | 91.37 | [196] |
| MgO | Pisonia alba | <100, spherical | UV/Vis spectroscopy, TEM, EDX, XRD, FTIR | DPPH, FRAP | - | 65, 69.3 | [197] |
| ZnO | Tecoma castanifolia | 70–75, spherical | UV/Vis spectroscopy, TEM, XRD, FTIR | DPPH | - | 56.11 | [198] |
| ZnO | Knoxia sumatrensis | 50–80, rod | UV/Vis spectroscopy, XRD, FTIR, FESEM | DPPH, ABTS•+, H2O2 | 95.80, 92.29, 98.92 | - 50.70 - | [199] |
| CuO | Cissus vitiginea | ~32.3, spherical | XRD, EDS, TEM | DPPH | 45.29 | 86.78 | [200] |
| TiO2 | Cola nitida | 25–191, spherical | UV/Vis, FTIR, TEM, EDX, XRD, FESEM | DPPH, H2O2 | - | 62.06 99.23 | [201] |
| Type | Composition | Advantages | References |
|---|---|---|---|
| Transfersomes | Lipid chains and surfactants “edge activators” | High elasticity, flexibility, and penetration potential | [243,244] |
| Ethosomes | Phospholipids, water, and high ethanol concentrations (20–45%) | High elasticity, permeability, distribution, flexibility, steric stability, and low aggregation | [245] |
| Niosomes | Non-ionic surfactant | Ease of production, low production cost, high chemical stability and storage | [246] |
| Cubosomes | Lipid cubic phase and coated by apolymer-based outer coating | High surface area, water solubility, better stability, and encapsulation efficiency | [247] |
| Biosomes | Bile salts | Deep penetration, high stability, and encapsulation efficiency | [248] |
| Novasomes | Numerous amphiphiles such as fatty alcohols and acids with each other, or with phospholipids | Precise targeting and efficient delivery | [249] |
| Vesosomes | Liposomal vesicles encapsulating smaller liposomes of different sizes | High protection and encapsulation efficiency | [250] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omran, B.; Baek, K.-H. Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights. Molecules 2021, 26, 7031. https://doi.org/10.3390/molecules26227031
Omran B, Baek K-H. Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights. Molecules. 2021; 26(22):7031. https://doi.org/10.3390/molecules26227031
Chicago/Turabian StyleOmran, Basma, and Kwang-Hyun Baek. 2021. "Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights" Molecules 26, no. 22: 7031. https://doi.org/10.3390/molecules26227031