Cholesteric Molecular Tweezer Artificial Receptor for Rapid and Highly Selective Detection of Ag+ in Food Samples
Abstract
:1. Introduction
2. Results and Discussion
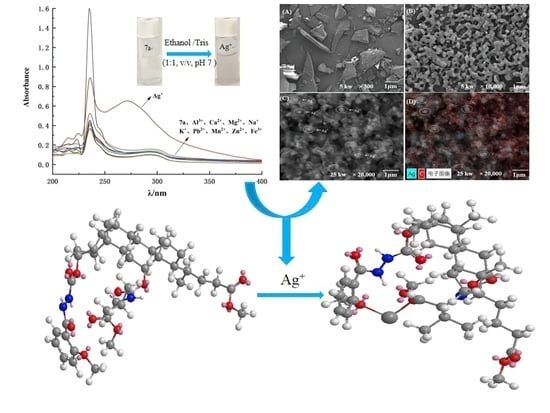
2.1. The Selectivity of Molecular Tweezer 7a to Ag+
2.2. Influence of Coexisting Ions and pH Value on the Recognition of Ag+ by Probe 7a
2.3. UV–Vis Spectral Titration Test and Job’s Plot Experiment
2.4. Microstructure Observation and Energy Spectrum Image Analysis
2.5. Analysis of the Mass Spectra and Infrared Spectra of the Complex
2.6. Results and Analysis of Nuclear Magnetic Titration Test
2.7. Computer Molecular Simulation
2.8. Quantitative Determination of Ag+ in Food Samples
3. Materials and Methods
3.1. Chemical Reagents and Instruments
3.2. Preparation of Cholesteric Molecular Tweezer Artificial Receptor 7a
3.3. General Procedure
3.4. Determination of Ag+ in Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Du, Z.L.; Chen, H.G.; Guo, X.Y.; Qin, L.; Lin, D.S.; Huo, L.L.; Yao, Y.Y.; Zhang, Z.H. Mechanism and industrial application feasibility analysis on microwave-assisted rapid synthesis of amino-carboxyl functionalized cellulose for enhanced heavy metal removal. Chemosphere 2021, 268, 128833. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Sun, D.W.; Pu, H.B.; Jayas, D.S. Determination of trace thiophanate-methyl and its metabolite carbendazim with teratogenic risk in red bell pepper (Capsicumannuum L.) by surface-enhanced Raman imaging technique. Food Chem. 2017, 218, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2914. [Google Scholar] [CrossRef]
- Hao, C.Y.; Zhao, X.M.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceuticals and personal-care products in environmental matrices. TRAC Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Richards, R.; Michael, E. Antibacterial action of silver nitrate. Biomed Lett. 1991, 45, 183–188. [Google Scholar]
- Song, J.; Kim, H.; Jang, Y.; Jang, J. Enhanced antibacterial activity of silver/polyrhodanine- composite decorated silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 11563–11568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Han, Z.X.; Fang, Z.H.; Shen, G.L.; Yu, R.Q. 5, 10, 15-Tris (pentafluorophenyl) corrole as highly selective neutral carrier for a silver ion-sensitive electrode. Anal. Chim. Acta 2006, 562, 210–215. [Google Scholar] [CrossRef]
- Vincent, K.P.; Andrew, B. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004, 30, 140–147. [Google Scholar]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 2010, 18, 89–108. [Google Scholar] [CrossRef]
- Klingelfus, T.; Lirola, J.R.; Silva, L.O.; Disner, G.R.; Vicentini, M.; Nadaline, M.J.B.; Robles, J.C.Z.; Trein, L.M.; Voigt, C.L.; Assis, H.C.S.; et al. Acute and long-term effects of trophic exposure to silver nanospheres in the central nervous system of a neotropical fish Hoplias intermedius. Neurotoxicology 2017, 63, 146–154. [Google Scholar] [CrossRef]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Houdt, R.V. Antimicrobial silver: Uses, toxicity and potential for resistance. Biometals 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Musil, S.; Kratzer, J.; Vobecky, M.; Benada, O.; Matousek, T. Silver chemical vapor generation for atomic absorption spectrometry: Minimization of transport losses, interferences and application to water analysis. J. Anal. Spectrom. 2010, 25, 1618–1626. [Google Scholar] [CrossRef]
- Wen, X.D.; Yang, S.C.; Zhang, H.Z.; Deng, Q.W. Combination of knotted reactor with portable tungsten coil electrothermal atomic absorption spectrometer for on-line determination of trace cadmium. Microchem. J. 2016, 124, 60–64. [Google Scholar] [CrossRef]
- Zaksas, N.P.; Gerasimova, V.A.; Nevinsky, G.A. Simultaneous determination of Fe, P, Ca, Mg, Zn, and Cu in whole blood by two-jet plasma atomic emission spectrometry. Talanta 2010, 80, 2187–2190. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, M.D.; Zachariadis, G.A.; Antemidis, A.N.; Stratis, J.A. Direct determination of toxic trace metals in honey and sugars using inductively coupled plasma atomic emission spectrometry. Talanta 2005, 65, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, N.; Sanagi, M.M.; Naim, A.A.; Ibrahim, W.A.W.; Baig, U. Dispersive micro-solid phase extraction method using newly pre-pared poly (methyl methacrylate) grafted agarose combined with ICP-MS for simultaneous determination of Cd, Ni, Cu and Zn in vegetable and natural water samples. Anal. Methods 2015, 7, 3215–3223. [Google Scholar] [CrossRef]
- Li, Y.; Peng, G.L.; Zhu, H. Dispersive liquid–liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim. Acta Part A 2015, 140, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Z.; Fierke, M.A.; Costa, R.C.; Gladysz, J.A.; Stein, A.; Bühlmann, P. Highly selective detection of silver in the low ppt range with ion-selective electrodes based on ionophore-doped fluorous membranes. Anal. Chem. 2010, 82, 7634–7640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojtaba, S.; Mehran, J.; Vito, L.; Alessandra, G.; Greta, D.F.; Mohammad, R.G.; Abdullah, Y. Novel Ag+ ion-selective electrodes based on two new mixed azathioether crowns containing a1, 10- phenanthroline sub-unit. Anal. Chim. Acta 2002, 462, 225–234. [Google Scholar]
- Jia, H.J.; Zhu, N.; Gao, Y.Y.; Wang, Y.Q.; Suo, Q.L. Effect of substituent structure of benzothiazole probe on recognition to metal ion. Spectrosc. Spectral Anal. 2020, 40, 3594–3598. [Google Scholar]
- Wang, F.; Nandhakumar, R.; Moon, J.H.; Kim, K.M.; Lee, J.Y.; Yoon, J.Y. Ratiometric fluorescent chemosensor for silver ion at physiological pH. Inorg. Chem. 2011, 50, 2240–2245. [Google Scholar] [CrossRef]
- Chen, X.Z.; Ma, X.D.; Wang, H.M.; Wang, M.; Zhang, Y.Y.; Liu, J.J.; Hou, S.C. A coumarin-based colorimetric and fluorescent dual probe for palladium (II) ions that can be used in live cells. New J. Chem. 2017, 41, 8026–8030. [Google Scholar] [CrossRef]
- Nath, S.; Maitra, U. A simple and general strategy for the design of fluorescent cation sensor beads. Org. Lett. 2006, 8, 3239–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Zhou, C.E. Recognition of anions by chiral unsymmetrical urea molecular clefts based on lithocholic acid. Chemistry 2009, 20, 265. [Google Scholar]
- Zhao, Z.G.; Liu, X.L.; Li, Q.H.; Chen, S.H. Progress in research on molecular tweezer artificial receptors. Chin. J. Org. Chem. 2009, 29, 1336–1353. [Google Scholar]
- Du, G.X.; Zhou, T.; Guo, M.L.; Huang, P.; Deng, Y.B.; Li, D.H. Development of Functional Phthalocyanine-Based Associate towards an Effective Fluorimetric Detection of Hg(II). Molecules 2018, 23, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.L.; Shi, Z.C. Microwave synthesis of steroids schiff base fluorescent probe and its recognition of metal ions. Anal. Lab. 2020, 39, 107–110. [Google Scholar]
- Thomas, K.G.; Thomas, K.J.; Das, S.; George, M.V. A squaraine-based near-infrared absorbing sensor for the selective detection of transition and other metal ions in aqueous media. Chem. Commun. 1997, 6, 597–598. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Li, C.; Shi, G.Q. Optically active supramolecular complexes of water-soluble achiral polythiophenes and folic acid: Spectroscopic studies and sensing applications. Langmuir 2008, 24, 12829–12835. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.Y.; Pan, D.; Li, H.W.; Yao, Y.H.; Lyu, Z.; Yang, L.T.; Ma, L.J. “Reactive” optical sensor for Hg2+ and its application in environmental aqueous media and biological systems. Anal. Bioanal. Chem. 2017, 409, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.X.; Wu, G.Y.; Li, Q.; Zhang, Y.M.; Wei, T.B.; Lin, Q.; Yao, H. Synthesis and Ag+ recognition performance of Pillar [5] arene based on water soluble column. J. Lanzhou Univ. Arts Sci. Nat. Sci. 2020, 34, 45–50. [Google Scholar]
- Zhang, X.; Fan, Y.; Zhan, T.G.; QI, Q.Y.; Zhao, X. A thiophene-derived hexaazatriphenylene (HAT) fluorescent sensor for the selective detection of Ag+ ion. Tetrahedron Lett. 2021, 68, 152911. [Google Scholar] [CrossRef]
- Sahu, M.; Manna, A.K.; Raut, K.; Mondal, J.; Patra, G.K. A highly selective thiosemicarbazone based Schiff base chemosensor for colorimetric detection of Cu2+ and Ag+ ions and turn-on fluorometric detection of Ag+ ions. Inorg. Chim. Acta 2020, 508, 119633. [Google Scholar] [CrossRef]
- Pandey, N.; Mehata, M.S.; Fatma, N.; Pant, S. Modulation of Fluorescence properties of 5-Aminoquinoline by Ag+ in aqueous media via charge transfer. J. Photochem. Photobiol. A 2020, 396, 112549. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wu, X.X.; Niu, Q.F.; Guo, Z.R.; Li, T.D.; Liu, H.X. Highly Selective and Sensitive Colorimetric and Fluorescent Chemosensor for Rapid Detection of Ag+, Cu2+ and Hg2+ Based on a Simple Schiff Base. J. Fluoresc. 2016, 27, 1–9. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Nunes, I.L.; Mercadante, A.Z.; Borsarelli, C.D. Photoprotection of Vitamins in Skimmed Milk by an Aqueous Soluble LycopeneGum Arabic Microcapsule. J. Agric. Food Chem. 2007, 55, 323–329. [Google Scholar] [CrossRef]
- Shi, P.Y.; Liu, L.L.; Li, X.R.; Zhao, Z.G.; Liu, X.L. Rapid and efficient synthesis of novel aryloxyacetyl hydrazone molecular tweezers in solvent-free conditions under microwave irradiation. Chin. J. Org. Chem. 2010, 6, 871–876. [Google Scholar]
- Ye, Y.; Suo, Y.R.; Yang, Y.J.; Yang, F.; Han, L.J. Application of UV spectrophotometry- NMR methodology in research on recognition properties of receptors based on deoxycholic acid for phenolic compounds. Anal. Test. Technol. Instrum. 2015, 21, 1–6. [Google Scholar]
- Ye, Y.; Suo, Y.R.; Yang, F.; Han, L.J. Microwave-assisted synthesis of novel chiral receptors derived from deoxycholic acid and their molecular recognition properties. Chem. Lett. 2014, 43, 1812–1814. [Google Scholar] [CrossRef]
- Shi, Z.C.; Zhao, Z.G. Synthesis of asymmetric bis schiff base fluorescnt probe and study on its recognition to metal ions. Mod. Chem. Ind. 2019, 39, 227–231. [Google Scholar]
- Kim, H.J.; Hong, J.; Hong, A.; Ham, S.; Lee, J.H.; Kim, J.S. Cu2+-induced inter-molecular static excimer formation of pyrenealkylamine. Org. Lett. 2008, 10, 1963–1966. [Google Scholar] [CrossRef] [PubMed]





 , NCH; ▴, 12α-COOCH3; •, ArOCH3).
, NCH; ▴, 12α-COOCH3; •, ArOCH3).
 , NCH; ▴, 12α-COOCH3; •, ArOCH3).
, NCH; ▴, 12α-COOCH3; •, ArOCH3).


| Metal Ions | Concentration (mol/L) | Relative Error/% | Metal Ions | Concentration (mol/L) | Relative Error/% |
|---|---|---|---|---|---|
| Al3+ | 4.5 × 10−5 | 9.5 | Mn2+ | 4.5 × 10−5 | −0.8 |
| Ca2+ | 4.5 × 10−5 | 0.6 | Zn2+ | 4.5 × 10−5 | 3.1 |
| Mg2+ | 4.5 × 10−5 | 3.5 | Fe3+ | 4.5 × 10−5 | −1.2 |
| Na+ | 4.5 × 10−5 | 9.7 | Pb2+ | 4.5 × 10−5 | 1.1 |
| K+ | 4.5 × 10−5 | −1 |
| Compound | Absorption Coefficient (L/mol·cm−1) | Linear Dynamic Range (mol/L) | Light Path Length of Sample Pool (cm) | Maximum Wavelength (nm) | Association Constant (L/mol) |
|---|---|---|---|---|---|
| 7a + Ag+ | 0.039 × 10−6 | 2 × 10−6 ~ 2 × 10−5 | 1 | 270 | 1.38 × 104 |
| Compound | Solvent | Detection Limit | Ref. |
|---|---|---|---|
 | Water | 1.25 × 10−5 M | [32] |
 | Chloroform | 1.79 × 10−6 M | [33] |
 | CH3OH/Tris-HCl | 1.6 × 10−6 M | [34] |
 | H2O | 5.3 × 10−5 M | [35] |
 | DMSO/H2O | 6.37 × 10−5 M | [36] |
 | Tris/HCl | 1 × 10−6 M | This work |
| Samples | New Method μg/g | RSD (%) | AAS Method μg/g | Relative Error (%) |
|---|---|---|---|---|
| milk powder | 19.44 ± 0.28 | 1.43 | 18.65 ± 0.80 | 4.24 |
| wheat flour | 31.06 ± 0.70 | 2.25 | 32.00 ± 0.38 | −2.93 |
| quinoa | 41.40 ± 0.47 | 1.14 | 40.20 ± 0.75 | 2.99 |
| wolfberry | 38.37 ± 0.27 | 0.72 | 37.58 ± 0.56 | 2.11 |
| Samples | Add (μg/g) | This Method (μg/g) | Recovery (%) | RSD (%, n = 3) | AAS Method (μg/g) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|---|---|
| quinoa | 10 | 51.52 ± 0.13 | 99 | 2.46 | 52.62 ± 0.20 | 103 | 2.29 |
| 40 | 85.33 ± 0.11 | 104 | 1.34 | 88.77 ± 0.20 | 109 | 3.86 | |
| wolfberry | 10 | 50.85 ± 0.12 | 103 | 2.35 | 46.75 ± 0.07 | 97 | 1.66 |
| 40 | 81.76 ± 0.20 | 104 | 2.50 | 74.23 ± 0.34 | 95 | 4.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ye, Y.; Wang, H.; Luo, L.-x. Cholesteric Molecular Tweezer Artificial Receptor for Rapid and Highly Selective Detection of Ag+ in Food Samples. Molecules 2021, 26, 6919. https://doi.org/10.3390/molecules26226919
Liu Z, Ye Y, Wang H, Luo L-x. Cholesteric Molecular Tweezer Artificial Receptor for Rapid and Highly Selective Detection of Ag+ in Food Samples. Molecules. 2021; 26(22):6919. https://doi.org/10.3390/molecules26226919
Chicago/Turabian StyleLiu, Zhe, Ying Ye, Hong Wang, and Li-xia Luo. 2021. "Cholesteric Molecular Tweezer Artificial Receptor for Rapid and Highly Selective Detection of Ag+ in Food Samples" Molecules 26, no. 22: 6919. https://doi.org/10.3390/molecules26226919