Ionization of Volatile Organics and Nonvolatile Biomolecules Directly from a Titanium Slab for Mass Spectrometric Analysis
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
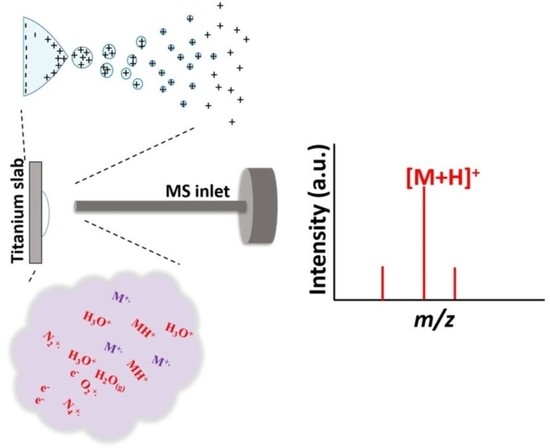
3.1. Examination of Titanium Slab-Based ASAI–MS Analysis through APCI
3.2. Gold-Coated Glass Slide-Based ASAI–MS Analysis
3.3. ASAI–MS Analysis for High-Volatility Analytes through APCI-Like Processes
3.4. Examination of the Lowest Detectable Concentration through the APCI Processe
3.5. Ionization Mechanism
3.6. Direct Analysis of Aroma Molecules from Plants by ASAI–MS Analysis
3.7. ASAI–MS Analysis of Small Organics and Biomolecules through ESI
3.8. Examination of the Lowest Detectable Concentration and Memory Effects through the ESI-Like Process
3.9. Application of ASAI–MS to the Analysis of Complex Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, D.I.; Dzidic, I.; Stillwell, R.N.; Haegele, K.D.; Horning, E.C. Atmospheric pressure ionization mass spectrometry. Corona discharge ion source for use in a liquid chromatograph-mass spectrometer-computer analytical system. Anal. Chem. 1975, 47, 2369–2373. [Google Scholar] [CrossRef]
- Yamashita, M.; Fenn, J.B. Electrospray ion source. Another variation on the free-jet theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Kebarle, P.; Tang, L. From ions in solution to ions in the gas phase-the mechanism of electrospray mass spectrometry. Anal. Chem. 1993, 65, 972A–986A. [Google Scholar] [CrossRef]
- Peschke, M.; Verkerk, U.H.; Kebarle, P. Features of the ESI mechanism that affect the observation of multiply charged noncovalent protein complexes and the determination of the association constant by the titration method. J. Am. Soc. Mass Spectrom. 2004, 15, 1424–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 30, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Cody, R.B.; Laramee, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Upton, K.T.; Schilling, K.A.; Beauchamp, J.L. Easily fabricated ion source for characterizing mixtures of organic compounds by direct analysis in real time mass spectrometry. Anal. Methods 2017, 9, 5065–5074. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.R.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef]
- Kauppila, T.; Kostiainen, R. Ambient mass spectrometry in the analysis of compounds of low polarity. Anal. Methods 2017, 9, 4936–4953. [Google Scholar] [CrossRef]
- McEwen, C.N.; McKay, R.G.; Larsen, B.S. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal. Chem. 2005, 77, 7826–7831. [Google Scholar] [CrossRef]
- Harris, G.A.; Galhena, A.S.; Fernandez, F.M. Ambient sampling/ionization mass spectrometry: Applications and current trends. Anal. Chem. 2011, 83, 4508–4538. [Google Scholar] [CrossRef] [PubMed]
- Shiea, J.; Huang, M.Z.; Hsu, H.J.; Lee, C.Y.; Yuan, C.H.; Beech, I.; Sunner, J. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun. Mass Spectrom. 2005, 19, 3701–3704. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, F.; Guo, C.; Zhang, S.; Zhang, X. Plasma-based ambient mass spectrometry: A step forward to practical applications. Anal. Methods 2017, 9, 4908–4923. [Google Scholar] [CrossRef]
- Haddad, R.; Sparrapan, R.; Eberlin, M.N. Desorption sonic spray ionization for (high) voltage-free ambient mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2901–2905. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, L.V.; Rutten, F.J.M.; Barrett, D.A.; Whitmore, T.; Seymour, D.; Greenwood, C.; Aranda, G.Y.; Robinson, S.; McCoustra, M. Surface analysis under ambient conditions using plasma-assisted desorption/ionization mass spectrometry. Anal. Chem. 2007, 79, 6094–6101. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chao, C.-S.; Mong, K.-K.T.; Chen, Y.-C. Ultrasonication-assisted spray ionization mass spectrometry for on-line monitoring of organic reactions. Chem. Commun. 2010, 46, 8347–8349. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.S.; Winkler, R. Design of a low-temperature plasma (LTP) probe with adjustable output temperature and variable beam diameter for the direct detection of organic molecules. Rapid Commun. Mass Spectrom. 2013, 27, 629–634. [Google Scholar] [CrossRef]
- Wang, B.; Trimpin, S. High-throughput solvent assisted ionization inlet for use in mass spectrometry. Anal. Chem. 2014, 86, 1000–1006. [Google Scholar] [CrossRef]
- Thomas, R.C.; Bruce, A.T.; Bradley, B.S. Atmospheric pressure ion sources. Mass Spectrom. Rev. 2009, 28, 870–897. [Google Scholar]
- Marek, S.; Przemyslaw, M.; Jerzy, S. Plasma-based ambient ionization mass spectrometry in bioanalytical sciences. Mass Spectrom. Rev. 2016, 35, 22–34. [Google Scholar]
- Cheng, S.C.; Jhang, S.S.; Huang, M.Z.; Shiea, J. Simultaneous detection of polar and nonpolar compounds by ambient mass spectrometry with a dual electrospray and atmospheric pressure chemical ionization source. Anal. Chem. 2015, 87, 1743–1748. [Google Scholar] [CrossRef]
- Brecht, D.; Uteschil, F.; Schmitz, O.J. Development of a fast-switching dual (ESI/APCI) ionization source for liquid chromatography/mass spectrometry. Rapid. Commun. Mass Spectrom. 2020, 34, e8845. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.J.; Cooks, R.G.; Ouyang, Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Development of a fast-switching dual (ESI/APCI) ionization source for liquid chromatography/mass spectrometry. Angew. Chem. Int. Ed. 2010, 49, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Wang, H.; Manicke, N.E.; Lin, J.M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.K.; Chen, Y.-C. Combination of Raman spectroscopy and mass spectrometry for online chemical analysis. Anal. Chem. 2016, 88, 9151–9157. [Google Scholar] [CrossRef]
- Meher, A.K.; Chen, Y.-C. Electrospray modifications for advancing mass spectrometric analysis. Mass Spectrom. 2017, 6, S0057. [Google Scholar] [CrossRef] [Green Version]
- Hsie, C.-H.; Chang, C.-H.; Urban, P.L.; Chen, Y.-C. Capillary action-supported contactless atmospheric pressure ionization for the combined sampling and mass spectrometric analysis of biomolecules. Anal. Chem. 2011, 83, 2866–2869. [Google Scholar] [CrossRef] [Green Version]
- Meher, A.K.; Chen, Y.-C. Polarization induced electrospray ionization mass spectrometry for the analysis of liquid, viscous and solid samples. J. Mass Spectrom. 2015, 50, 444–450. [Google Scholar] [CrossRef]
- Goldman, M.; Goldman, A.; Sigmond, R.S. He corona discharge, its properties and specific uses. Pure Appl. Chem. 1985, 57, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-L.; Chen, T.-Y.; Chen, Y.-C. Carbon fiber ionization mass spectrometry for the analysis of analytes in vapor, liquid, and solid phases. Anal. Chem. 2017, 89, 13458–13465. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Chen, Y.-C. Reactive carbon fiber ionization-mass spectrometry for characterization of unsaturated hydrocarbons from plant aroma. Anal. Bioanal. Chem. 2020, 412, 5489–5497. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.-Y.; Wang, M.-J.; Wu, J.-J.; Chen, Y.-C. Ionization of Volatile Organics and Nonvolatile Biomolecules Directly from a Titanium Slab for Mass Spectrometric Analysis. Molecules 2021, 26, 6760. https://doi.org/10.3390/molecules26226760
Huang D-Y, Wang M-J, Wu J-J, Chen Y-C. Ionization of Volatile Organics and Nonvolatile Biomolecules Directly from a Titanium Slab for Mass Spectrometric Analysis. Molecules. 2021; 26(22):6760. https://doi.org/10.3390/molecules26226760
Chicago/Turabian StyleHuang, De-Yi, Meng-Jiy Wang, Jih-Jen Wu, and Yu-Chie Chen. 2021. "Ionization of Volatile Organics and Nonvolatile Biomolecules Directly from a Titanium Slab for Mass Spectrometric Analysis" Molecules 26, no. 22: 6760. https://doi.org/10.3390/molecules26226760