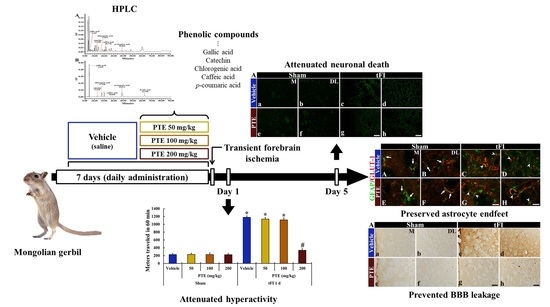
Populus tomentiglandulosa Extract Is Rich in Polyphenols and Protects Neurons, Astrocytes, and the Blood-Brain Barrier in Gerbil Striatum Following Ischemia-Reperfusion Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Populus Tomentiglandulosa Extract (PTE)
2.2. Qualitative Analysis of PTE
2.3. Protocol, Experimental Animals and Groups
2.4. Induction of tFI
2.5. Spontaneous Motor Activity (SMA) Test
2.6. Preparation of Brain Sections for Histological Observation
2.7. Cresyl Violet (CV) Histochemistry
2.8. Histofluorescence with Fluoro-Jade B (F-JB)
2.9. Immunohistochemistry (IHC)
2.10. Double Immunofluorescence
2.11. Statistical Analysis
3. Results
3.1. PTE Contained Phenolic Compounds
3.2. PTE 200 mg/kg Was Significantly Effective on tFI-induced Locomotor Activity
3.3. CV Stainability after tFI Was Conserved by PTE 200 mg/kg
3.4. F-JB+ (Dead) Cells Following tFI Were Significantly Reduced by PTE 200 mg/kg
3.5. PTE 200 mg/kg Protected tFI-induced Damage of Astrocytes
3.6. PTE 200 mg/kg Preserved AEF from tFI Injury
3.7. IgG Leakage Following tFI Was Protected by PTE 200 mg/kg
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line hplc-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- Debbache-Benaida, N.; Atmani-Kilani, D.; Schini-Keirth, V.B.; Djebbli, N.; Atmani, D. Pharmacological potential of Populus nigra extract as antioxidant, anti-inflammatory, cardiovascular and hepatoprotective agent. Asian Pac. J. Trop. Biomed. 2013, 3, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Thuong, P.T.; Min, B.S.; Ngoc, T.M.; Hung, T.M.; Lee, I.S.; Na, M.; Seong, Y.H.; Song, K.S.; Bae, K. Phenolic glycosides with antioxidant activity from the stem bark of Populus davidiana. J. Nat. Prod. 2006, 69, 1370–1373. [Google Scholar] [CrossRef]
- Harbilas, D.; Brault, A.; Vallerand, D.; Martineau, L.C.; Saleem, A.; Arnason, J.T.; Musallam, L.; Haddad, P.S. Populus balsamifera L. (salicaceae) mitigates the development of obesity and improves insulin sensitivity in a diet-induced obese mouse model. J. Ethnopharmacol. 2012, 141, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, T.K.; Ahn, J.H.; Shin, B.N.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Kim, J.D.; Lee, Y.J.; Kang, I.J.; et al. Pre-treated Populus tomentiglandulosa extract inhibits neuronal loss and alleviates gliosis in the gerbil hippocampal ca1 area induced by transient global cerebral ischemia. Anat. Cell Biol. 2017, 50, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, T.; Kato, H.; Kogure, K. Selective neuronal vulnerability following transient cerebral ischemia in the gerbil: Distribution and time course. Acta Neurol. Scand. 1989, 80, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Mhadu, N.H.; Al-Dalain, S.M.; Martínez, G.; León, O.S. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci. Res. 2001, 41, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, T.K.; Kim, D.W.; Sim, H.; Lee, J.C.; Kim, J.D.; Ahn, J.H.; Lee, C.H.; Kim, Y.M.; Won, M.H.; et al. Neuroprotective effects of salicin in a gerbil model of transient forebrain ischemia by attenuating oxidative stress and activating pi3k/akt/gsk3beta pathway. Antioxidants 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, T.K.; Park, C.W.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.C.; Yang, G.E.; Kim, J.D.; Shin, M.C.; et al. Pycnogenol((r)) supplementation attenuates memory deficits and protects hippocampal ca1 pyramidal neurons via antioxidative role in a gerbil model of transient forebrain ischemia. Nutrients 2020, 12, 2477. [Google Scholar] [CrossRef]
- Park, C.W.; Ahn, J.H.; Lee, T.K.; Park, Y.E.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Park, Y.; Cho, J.H.; et al. Post-treatment with oxcarbazepine confers potent neuroprotection against transient global cerebral ischemic injury by activating nrf2 defense pathway. Biomed. Pharmacother. 2020, 124, 109850. [Google Scholar] [CrossRef] [PubMed]
- Mayevsky, A.; Breuer, Z. Brain vasculature and mitochondrial responses to ischemia in gerbils. I. Basic anatomical patterns and biochemical correlates. Brain Res. 1992, 598, 242–250. [Google Scholar] [CrossRef]
- Ohk, T.G.; Yoo, K.Y.; Park, S.M.; Shin, B.N.; Kim, I.H.; Park, J.H.; Ahn, H.C.; Lee, Y.J.; Kim, M.J.; Kim, T.Y.; et al. Neuronal damage using fluoro-jade b histofluorescence and gliosis in the striatum after various durations of transient cerebral ischemia in gerbils. Neurochem. Res. 2012, 37, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.K.; Yoo, K.Y.; Shin, B.N.; Kim, I.H.; Park, J.H.; Lee, C.H.; Choi, J.H.; Cho, Y.J.; Kang, I.J.; Kim, Y.M.; et al. Neuronal damage in hippocampal subregions induced by various durations of transient cerebral ischemia in gerbils using fluoro-jade b histofluorescence. Brain Res. 2012, 1437, 50–57. [Google Scholar] [CrossRef]
- Kiss, J.P.; Zsilla, G.; Vizi, E.S. Inhibitory effect of nitric oxide on dopamine transporters: Interneuronal communication without receptors. Neurochem. Int. 2004, 45, 485–489. [Google Scholar] [CrossRef]
- Yoshioka, H.; Niizuma, K.; Katsu, M.; Sakata, H.; Okami, N.; Chan, P.H. Consistent injury to medium spiny neurons and white matter in the mouse striatum after prolonged transient global cerebral ischemia. J. Neurotrauma 2011, 28, 649–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulsinelli, W.A.; Brierley, J.B.; Plum, F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982, 11, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Crain, B.J.; Westerkam, W.D.; Harrison, A.H.; Nadler, J.V. Selective neuronal death after transient forebrain ischemia in the mongolian gerbil: A silver impregnation study. Neuroscience 1988, 27, 387–402. [Google Scholar]
- Terashima, T.; Namura, S.; Hoshimaru, M.; Uemura, Y.; Kikuchi, H.; Hashimoto, N. Consistent injury in the striatum of c57bl/6 mice after transient bilateral common carotid artery occlusion. Neurosurgery 1998, 43, 900–907. [Google Scholar] [CrossRef]
- Zhu, J.C.; Si, M.Y.; Li, Y.Z.; Chen, H.Z.; Fan, Z.C.; Xie, Q.D.; Jiao, X.Y. Circulating tight junction proteins mirror blood-brain barrier integrity in leukaemia central nervous system metastasis. Hematol. Oncol. 2017, 35, 365–373. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef] [Green Version]
- Kassner, A.; Merali, Z. Assessment of blood-brain barrier disruption in stroke. Stroke 2015, 46, 3310–3315. [Google Scholar] [CrossRef]
- Maeda, M.; Akai, F.; Nishida, S.; Yanagihara, T. Intracerebral distribution of albumin after transient cerebral ischemia: Light and electron microscopic immunocytochemical investigation. Acta Neuropathol. 1992, 84, 59–66. [Google Scholar] [CrossRef]
- Lan, X.B.; Wang, Q.; Yang, J.M.; Ma, L.; Zhang, W.J.; Zheng, P.; Sun, T.; Niu, J.G.; Liu, N.; Yu, J.Q. Neuroprotective effect of vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharm. 2019, 118, 109196. [Google Scholar] [CrossRef] [PubMed]
- Michalski, D.; Grosche, J.; Pelz, J.; Schneider, D.; Weise, C.; Bauer, U.; Kacza, J.; Gartner, U.; Hobohm, C.; Hartig, W. A novel quantification of blood-brain barrier damage and histochemical typing after embolic stroke in rats. Brain Res. 2010, 1359, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.L.; Lu, L.Q.; Li, W.; Lou, Q.; Guo, H.G.; Shi, Q.J. Oral administration of ampelopsin protects against acute brain injury in rats following focal cerebral ischemia. Exp. Med. 2017, 13, 1725–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-K.; Park, J.H.; Ahn, J.H.; Kim, H.; Song, M.; Lee, J.-C.; Dai Kim, J.; Jeon, Y.H.; Choi, J.H.; Lee, C.H. Pretreatment of Populus tomentiglandulosa protects hippocampal ca1 pyramidal neurons from ischemia-reperfusion injury in gerbils via increasing sods expressions and maintaining bdnf and igf-i expressions. Chin. J. Nat. Med. 2019, 17, 424–434. [Google Scholar] [CrossRef]
- Choi, S.-I.; Hwang, S.-J.; Lee, O.-H.; Kim, J.D. Antioxidant activity and component analysis of Populus tomentiglandulosa extract. Korean J. Food Sci. Technol. 2020, 52, 119–124. [Google Scholar]
- Her, Y.; Lee, T.K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.C.; Ahn, J.H.; Park, J.H.; Lee, J.W.; Hong, J.; et al. Topical application of aronia melanocarpa extract rich in chlorogenic acid and rutin reduces uvb-induced skin damage via attenuating collagen disruption in mice. Molecules 2020, 25, 4577. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chen, B.H.; Park, J.H.; Shin, B.N.; Lee, T.K.; Cho, J.H.; Lee, J.C.; Park, J.R.; Yang, S.R.; Ryoo, S.; et al. Early iv-injected human dermis-derived mesenchymal stem cells after transient global cerebral ischemia do not pass through damaged blood-brain barrier. J. Tissue Eng. Regen. Med. 2018, 12, 1646–1657. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar] [PubMed]
- Park, J.H.; Kim, D.W.; Lee, T.K.; Park, C.W.; Park, Y.E.; Ahn, J.H.; Lee, H.A.; Won, M.H.; Lee, C.H. Improved hcn channels in pyramidal neurons and their new expression levels in pericytes and astrocytes in the gerbil hippocampal ca1 subfield following transient ischemia. Int. J. Mol. Med. 2019, 44, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Park, J.H.; Ahn, J.H.; Kim, I.H.; Cho, J.H.; Choi, J.H.; Yoo, K.Y.; Lee, C.H.; Hwang, I.K.; Cho, J.H.; et al. New gabaergic neurogenesis in the hippocampal ca1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016, 26, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-jade b: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.J.; Miller, K.M.; Fugaccia, I.; Scheff, S.W. Regional distribution of fluoro-jade b staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005, 193, 125–130. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, B.N.; Chen, B.H.; Kim, I.H.; Ahn, J.H.; Cho, J.H.; Tae, H.J.; Lee, J.C.; Lee, C.H.; Kim, Y.M.; et al. Neuroprotection and reduced gliosis by atomoxetine pretreatment in a gerbil model of transient cerebral ischemia. J. Neurol. Sci. 2015, 359, 373–380. [Google Scholar] [CrossRef]
- Paizs, M.; Engelhardt, J.I.; Siklos, L. Quantitative assessment of relative changes of immunohistochemical staining by light microscopy in specified anatomical regions. J. Microsc. 2009, 234, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, F.; Rovella, V.; Pastore, D.; Bellia, A.; Abete, P.; Donadel, G.; Santini, S.; Beck, H.; Ricordi, C.; Daniele, N.D.; et al. Polyphenols and ischemic stroke: Insight into one of the best strategies for prevention and treatment. Nutrients 2021, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Jhelum, P.; Wahul, A.B.; Kamle, A.; Kumawat, S.; Kumar, A.; Bhutani, K.K.; Tripathi, S.M.; Chakravarty, S. Sameerpannag ras mixture (srm) improved neurobehavioral deficits following acute ischemic stroke by attenuating neuroinflammatory response. J. Ethnopharmacol. 2017, 197, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Simonyi, A.; Wang, Q.; Miller, R.L.; Yusof, M.; Shelat, P.B.; Sun, A.Y.; Sun, G.Y. Polyphenols in cerebral ischemia: Novel targets for neuroprotection. Mol. Neurobiol. 2005, 31, 135–147. [Google Scholar] [CrossRef]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Johnston, S.C.; Mendis, S.; Mathers, C.D. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009, 8, 345–354. [Google Scholar] [CrossRef]
- Restivo, L.; Middei, S.; Mingfu, L.; Reggio, R.; Passino, E.; Formisano, R.; Ammassari-Teule, M. Potentiation of ischemia-related behavioral alterations by electro-acupuncture in gerbils. Funct Neurol. 2004, 19, 19–23. [Google Scholar] [PubMed]
- Araki, H.; Yamamoto, T.; Kobayashi, Y.; Futagami, K.; Kawasaki, H.; Gomita, Y. Effect of methamphetamine and imipramine on cerebral ischemia-induced hyperactivity in mongolian gerbils. Jpn. J. Pharm. 2002, 88, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Katsuta, K.; Umemura, K.; Ueyama, N.; Matsuoka, N. Pharmacological evidence for a correlation between hippocampal ca1 cell damage and hyperlocomotion following global cerebral ischemia in gerbils. Eur. J. Pharm. 2003, 467, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Dirnagl, U. Bench to bedside: The quest for quality in experimental stroke research. J. Cereb. Blood Flow Metab. 2006, 26, 1465–1478. [Google Scholar] [CrossRef] [Green Version]
- Spray, S.; Edvinsson, L. Improved assessment of outcomes following transient global cerebral ischemia in mice. Exp. Brain Res. 2016, 234, 1925–1934. [Google Scholar] [CrossRef]
- Harukuni, I.; Bhardwaj, A. Mechanisms of brain injury after global cerebral ischemia. Neurol. Clin. 2006, 24, 1–21. [Google Scholar] [CrossRef]
- Horner, P.J.; Palmer, T.D. New roles for astrocytes: The nightlife of an ‘astrocyte’. La vida loca! Trends Neurosci. 2003, 26, 597–603. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect Biol. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.F.; Blomstrand, F.; Blomstrand, C.; Eriksson, P.S.; Nilsson, M. Astrocytes and stroke: Networking for survival? Neurochem. Res. 2003, 28, 293–305. [Google Scholar] [CrossRef]
- Bylicky, M.A.; Mueller, G.P.; Day, R.M. Mechanisms of endogenous neuroprotective effects of astrocytes in brain injury. Oxid. Med. Cell Longev. 2018, 2018, 6501031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Park, J.H.; Shin, M.C.; Cho, J.H.; Lee, T.K.; Kim, H.; Song, M.; Park, C.W.; Park, Y.E.; Lee, J.C.; et al. Fate of astrocytes in the gerbil hippocampus after transient global cerebral ischemia. Int. J. Mol. Sci. 2019, 20, 845. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraig, R.P.; Dong, L.M.; Thisted, R.; Jaeger, C.B. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J. Neurosci. 1991, 11, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Lascola, C.; Kraig, R.P. Astroglial acid-base dynamics in hyperglycemic and normoglycemic global ischemia. Neurosci. Biobehav. Rev. 1997, 21, 143–150. [Google Scholar] [CrossRef]
- Lee, C.H.; Ahn, J.H.; Lee, T.K.; Sim, H.; Lee, J.C.; Park, J.H.; Shin, M.C.; Cho, J.H.; Kim, D.W.; Won, M.H.; et al. Comparison of neuronal death, blood-brain barrier leakage and inflammatory cytokine expression in the hippocampal ca1 region following mild and severe transient forebrain ischemia in gerbils. Neurochem. Res. 2021. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, J.H.; Cho, J.H.; Park, J.H.; Ahn, J.H.; Tae, H.J.; Cho, G.S.; Yan, B.C.; Hwang, I.K.; Lee, C.H.; et al. Impact of hyperthermia before and during ischemia-reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J. Neurol. Sci. 2015, 348, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Lee, T.K.; Kang, I.J.; Sim, H.; Lee, J.C.; Ahn, J.H.; Kim, D.W.; Park, J.H.; Lee, C.H.; Kim, J.D.; Won, M.H.; et al. Therapeutic effects of decursin and angelica gigas nakai root extract in gerbil brain after transient ischemia via protecting bbb leakage and astrocyte endfeet damage. Molecules 2021, 26, 2161. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Tepper, D.; Leonard, A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front. Neurol. 2013, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.Q.; Wang, S.; Kim, H.Y.; Storrie, H.; Rosen, B.R.; Mooney, D.J.; Wang, X.; Lo, E.H. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 2006, 12, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Park, J.H.; Ahn, J.H.; Kim, J.D.; Cho, J.H.; Lee, T.K.; Won, M.H. Stronger antioxidant enzyme immunoreactivity of Populus tomentiglandulosa extract than ascorbic acid in rat liver and kidney. Iran. J. Basic Med. Sci. 2019, 22, 963–967. [Google Scholar] [PubMed]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Yang, C.; DeMars, K.M.; Alexander, J.C.; Febo, M.; Candelario-Jalil, E. Sustained neurological recovery after stroke in aged rats treated with a novel prostacyclin analog. Stroke 2017, 48, 1948–1956. [Google Scholar] [CrossRef]








| PTE (mg/g) | |
|---|---|
| Gallic acid | 1.4 ± 0.35 |
| Catechin | 9.1 ± 0.27 |
| Chlorogenic acid | 1.6 ± 0.86 |
| Caffeic acid | 4.1 ± 0.57 |
| p-coumaric acid | 2.1 ± 0.47 |
| Ferulic acid | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-K.; Lee, J.-C.; Kim, J.-D.; Kim, D.-W.; Ahn, J.-H.; Park, J.-H.; Kim, H.-I.; Cho, J.-H.; Choi, S.-Y.; Won, M.-H.; et al. Populus tomentiglandulosa Extract Is Rich in Polyphenols and Protects Neurons, Astrocytes, and the Blood-Brain Barrier in Gerbil Striatum Following Ischemia-Reperfusion Injury. Molecules 2021, 26, 5430. https://doi.org/10.3390/molecules26185430
Lee T-K, Lee J-C, Kim J-D, Kim D-W, Ahn J-H, Park J-H, Kim H-I, Cho J-H, Choi S-Y, Won M-H, et al. Populus tomentiglandulosa Extract Is Rich in Polyphenols and Protects Neurons, Astrocytes, and the Blood-Brain Barrier in Gerbil Striatum Following Ischemia-Reperfusion Injury. Molecules. 2021; 26(18):5430. https://doi.org/10.3390/molecules26185430
Chicago/Turabian StyleLee, Tae-Kyeong, Jae-Chul Lee, Jong-Dai Kim, Dae-Won Kim, Ji-Hyeon Ahn, Joon-Ha Park, Hyung-Il Kim, Jun-Hwi Cho, Soo-Young Choi, Moo-Ho Won, and et al. 2021. "Populus tomentiglandulosa Extract Is Rich in Polyphenols and Protects Neurons, Astrocytes, and the Blood-Brain Barrier in Gerbil Striatum Following Ischemia-Reperfusion Injury" Molecules 26, no. 18: 5430. https://doi.org/10.3390/molecules26185430