Perturbating Intramolecular Hydrogen Bonds through Substituent Effects or Non-Covalent Interactions
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. HOCH2(CH2)nCH2NH2 (n = 0–5) Compounds
3.2. HOCHX(CH2)nCH2NH2 and HOCH2(CH2)nCHXNH2 (n = 0–5, X = F, Cl, Br) Derivatives
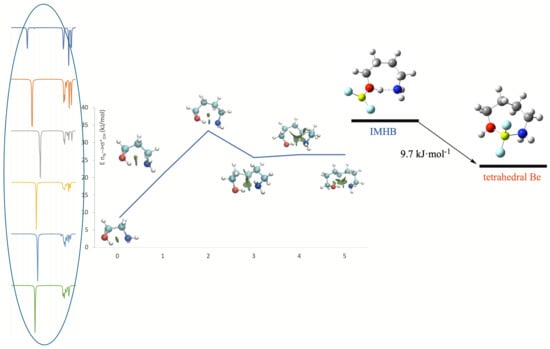
3.3. Complexes between HOCH2(CH2)nCH2NH2 (n = 0–3) and BeF2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pauling, L. The Shared-Electron Chemical Bond. Proc. Natl. Acad. Sci. USA 1928, 14, 359–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latimer, W.M.; Rodebush, W.H. Polarity and Ionization from the Standpoint of the Lewis Theory of Valence. J. Am. Chem. Soc. 1920, 42, 1419–1433. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L.; Brockway, L.O. The Structure of the Carboxyl Group: I. The Investigation of Formic Acid by the Diffraction of Electrons. Proc. Natl. Acad. Sci. USA 1934, 20, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, G.C.; McClelland, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, CA, USA, 1960. [Google Scholar]
- Kollman, P.A.; Allen, L.C. Theory of hydrogen bond.Electronic structure and properties of water dimer. J. Chem. Phys. 1969, 51, 3286. [Google Scholar] [CrossRef]
- Witkowski, A. Far-Infrared Spectrum of the Hydrogen Bond in Acetic Acid Dimers. J. Chem. Phys. 1970, 52, 4403. [Google Scholar] [CrossRef]
- Allen, L.C. Simple model of hydrogen bonding. J. Am. Chem. Soc. 1975, 97, 6921–6940. [Google Scholar] [CrossRef]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer Science and Business Media LLC: Berlin, Germany, 1991. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Grabowski, S.J. Hydrogen Bonding—New Insights; Springer: Dordrecht, The Netherlands, 2006; Volume 3. [Google Scholar]
- Han, K.-L.; Zhao, G.-J. Hydrogen Bonding and Transfer in the Excited State; John Wiley& Sons: Chichester, UK, 2011. [Google Scholar]
- Kuo, S.-W. Hydrogen Bonding in Polymeric Materials; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Han, K.; Zhao, G. Hydrogen-Bonding Research in Photochemistry, Photobiology, and Optoelectronic Materials; World Scientific Pub. Co. Pte. Lt.: Singapore, 2018. [Google Scholar]
- Grabowski, S.J. Understanding Hydrogen Bonds: Theoretical and Experimental Views; CPI Group, UK Ltd.: Croydon, UK, 2020. [Google Scholar]
- Sidwick, N.V.; Callow, R.K. Abnormal benzene derivatives. J. Chem. Soc. 1924, 125, 527–538. [Google Scholar] [CrossRef]
- Amstrong, H.E. Bigamous Hydrogen—A Protest. Nature 1926, 117, 553–554. [Google Scholar] [CrossRef]
- Lowry, T.M. Optical Rotatory Dispersion. Nature 1926, 117, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Buemi, G.; Zuccarello, F. DFT study of the intramolecular hydrogen bonds in the amino and nitro-derivatives of malonaldehyde. Chem. Phys. 2004, 306, 115–129. [Google Scholar] [CrossRef]
- Gonzalez, L.; Mó, O.; Yáñez, M. Protonation and Deprotonation of Thiomalonaldehyde. The Role of the Intramolecular Hydrogen Bond. In Recent Theoretical and Experimental Advances in Hydrogen Bonded Clusters; Springer Science and Business Media LLC: Berlin, Germany, 2000; pp. 393–402. [Google Scholar]
- West-Nielsen, M.; Dominiak, P.; Wozniak, K.; Hansen, P.E. Strong intramolecular hydrogen bonding involving nitro- and acetyl groups. Deuterium isotope effects on chemical shifts. J. Mol. Struct. 2006, 789, 81–91. [Google Scholar] [CrossRef]
- Nazarparvar, E.; Zahedi, M.; Klein, E. Density Functional Theory (B3LYP) Study of Substituent Effects on O–H Bond Dissociation Enthalpies of trans-Resveratrol Derivatives and the Role of Intramolecular Hydrogen Bonds. J. Org. Chem. 2012, 77, 10093–10104. [Google Scholar] [CrossRef]
- Nazarparvar, E.; Zahedi, M.; Klein, E. Theoretical study of the substituent effects on O–H BDE of trans-resveratrol derivatives in water and benzene: NBO analysis of intramolecular hydrogen bonds. Struct. Chem. 2014, 26, 47–59. [Google Scholar] [CrossRef]
- Galvao, T.L.P.; Rocha, I.M.; da Silva, M.D.M.C.R.; da Silva, M.A.V.R. Energetic Study of 4(3H)-Pyrimidinone: Aromaticity of Reactions, Hydrogen Bond Rules, and Support for an Anomeric Effect. J. Phys. Chem. A 2014, 118, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Hu, J.-W.; Huang, T.-H.; Chen, K.-Y.; Chou, P.-T. Excited-state intramolecular proton transfer in the kinetic-control regime. Phys. Chem. Chem. Phys. 2020, 22, 22271–22278. [Google Scholar] [CrossRef]
- Laner, J.N.; Junior, H.d.C.S.; Rodembusch, F.S.; Moreira, E.C. New insights on the ESIPT process based on solid-state data and state-of-the-art computational methods. Phys. Chem. Chem. Phys. 2021, 23, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ji, D.; Zhao, J. Theoretical insights into photochemical behavior and ESIPT mechanism for 2,6-dimethyl phenyl derivatives. Chem. Phys. Lett. 2021, 767, 138377. [Google Scholar] [CrossRef]
- Hirahara, M.; Nakano, H.; Uchida, K.; Yamamoto, R.; Umemura, Y. Intramolecular Hydrogen Bonding: A Key Factor Controlling the Photosubstitution of Ruthenium Complexes. Inorg. Chem. 2020, 59, 11273–11286. [Google Scholar] [CrossRef] [PubMed]
- Listkowski, A.; Masiera, N.; Kijak, M.; Luboradzki, R.; Leśniewska, B.; Waluk, J. Controlling Emissive Properties by Intramolecular Hydrogen Bonds: Alkyl and Aryl meso-Substituted Porphycenes. Chem. A Eur. J. 2021, 27, 6324–6333. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Q.; Wang, X.; Wang, M.; Wang, Q.; Shen, H.-M.; Yang, Y.-F.; She, Y.-B. Intramolecular hydrogen bond-induced high chemical stability of metal–organic frameworks. Inorg. Chem. Front. 2020, 7, 3548–3554. [Google Scholar] [CrossRef]
- Li, W.; Chasing, P.; Benchaphanthawee, W.; Nalaoh, P.; Chawanpunyawat, T.; Kaiyasuan, C.; Kungwan, N.; Namuangruk, S.; Sudyoadsuk, T.; Promarak, V. Intramolecular hydrogen bond—Enhanced electroluminescence performance of hybridized local and charge transfer (HLCT) excited-state blue-emissive materials. J. Mater. Chem. C 2021, 9, 497–507. [Google Scholar] [CrossRef]
- Avramopoulos, A.; Jabłoński, M.; Papadopoulos, M.G.; Sadlej, A.J. Linear and nonlinear electric properties and their dependence on the conformation and intramolecular H-bonding: A model study. Chem. Phys. 2006, 328, 33–44. [Google Scholar] [CrossRef]
- Fallas, J.A.; Gonzalez, L.; Corral, I. Density functional theory rationalization of the substituent effects in trifluoromethyl-pyridinol derivatives. Tetrahedron 2009, 65, 232–239. [Google Scholar] [CrossRef]
- Syrén, P.-O.; Le Joubioux, F.; Henda, Y.B.; Maugard, T.; Hult, K.; Graber, M. Proton Shuttle Mechanism in the Transition State of Lipase-Catalyzed N-Acylation of Amino Alcohols. ChemCatChem 2013, 5, 1842–1853. [Google Scholar] [CrossRef] [Green Version]
- Loru, D.; Pena, I.; Alonso, J.L.; Sanz, M.E. Intramolecular interactions in the polarheadgroup of sphingosine: Serinol. Chem. Commun. 2016, 52, 3615–3618. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhong, J.; Zhang, Q.; Qian, J.; Song, K.; Ruan, C.; Xu, J.; Ding, K.; Zhang, J. Rational Design and Structure Validation of a Novel Peptide Inhibitor of the Adenomatous-Polyposis-Coli (APC)–Rho-Guanine-Nucleotide-Exchange-Factor-4 (Asef) Interaction. J. Med. Chem. 2018, 61, 8017–8028. [Google Scholar] [CrossRef]
- Giubertoni, G.; Sofronov, O.O.; Bakker, H.J. Effect of intramolecular hydrogen-bond formation on the molecular conformation of amino acids. Commun. Chem. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Jabłoński, M. Binding of X–H to the lone-pair vacancy: Charge-inverted hydrogen bond. Chem. Phys. Lett. 2009, 477, 374–376. [Google Scholar] [CrossRef]
- Jabłoński, M. QTAIM-Based Comparison of Agostic Bonds and Intramolecular Charge-Inverted Hydrogen Bonds. J. Phys. Chem. A 2015, 119, 4993–5008. [Google Scholar] [CrossRef]
- Penn, R.E.; Curl, R.F. Microwave Spectrum of 2-Aminoethanol: Structural Effects of the Hydrogen Bond. J. Chem. Phys. 1971, 55, 651–658. [Google Scholar] [CrossRef]
- Kelterer, A.-M.; Ramek, M. Intramolecular hydrogen bonding in 2-aminoethanol, 3-aminopropanol and 4-aminobutanol. J. Mol. Struct. Theochem 1991, 232, 189–201. [Google Scholar] [CrossRef]
- Khalil, A.S.; Lavrich, R.J. Intramolecular hydrogen bond stabilized conformation of 3-aminopropanol. J. Mol. Spectrosc. 2020, 370, 111279. [Google Scholar] [CrossRef]
- Khalil, A.S.; Duguay, T.M.; Lavrich, R.J. Conformation and hydrogen bonding in 4-Aminobutanol. J. Mol. Struct. 2017, 1138, 12–16. [Google Scholar] [CrossRef]
- Batista, P.R.; Karas, L.J.; Viesser, R.V.; De Oliveira, C.C.; Gonçalves, M.B.; Tormena, C.F.; Rittner, R.; Ducati, L.C.; De Oliveira, P.R. Dealing with Hydrogen Bonding on the Conformational Preference of 1,3-Aminopropanols: Experimental and Molecular Dynamics Approaches. J. Phys. Chem. A 2019, 123, 8583–8594. [Google Scholar] [CrossRef]
- Yáñez, M.; Sanz, P.; Mó, O.; Alkorta, I.; Elguero, J. Beryllium Bonds, do they exist? J. Chem Theor. Comput. 2009, 5, 2763–2771. [Google Scholar] [CrossRef]
- Perera, L.C.; Raymond, O.; Henderson, W.; Brothers, P.J.; Plieger, P.G. Advances in beryllium coordination chemistry. Coord. Chem. Rev. 2017, 352, 264–290. [Google Scholar] [CrossRef]
- Li, Q.Z.; Liu, X.F.; Li, R.; Cheng, J.B.; Li, W.Z. Competition between dihydrogen bond and beryllium bond in complexes between HBeH and HArF: A huge blue shift of distant H-Ar stretch. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 135–140. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Li, Q.; Cheng, J.; Xiao, B.; Yu, X. Modulating the strength of tetrel bonding through beryllium bonding. J. Mol. Model. 2016, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Braunschweig, H.; Ewing, W.; Kramer, T.; Mies, J.; Schuster, J.K. Beryllium bis(diazaborolyl): Old neighbors finally shake hands. Chem. Commun. 2014, 51, 737–740. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Zhou, M.; Andrada, D.M.; Frenking, G. Experimental and Theoretical Studies of the Infrared Spectra and Bonding Properties of NgBeCO3and a Comparison with NgBeO (Ng = He, Ne, Ar, Kr, Xe). J. Phys. Chem. A 2014, 119, 2543–2552. [Google Scholar] [CrossRef]
- Albrecht, L.; Boyd, R.J.; Mó, O.; Yáñez, M. Cooperativity between hydrogen bonds and beryllium bonds in (H2O)nBeX2 (n = 1–3, X = H, F) complexes. A new perspective. Phys. Chem. Chem. Phys. 2012, 14, 14540–14547. [Google Scholar] [CrossRef]
- Mó, O.; Yáñez, M.; Alkorta, I.; Elguero, J. Modulating the Strength of Hydrogen Bonds through Beryllium Bonds. J. Chem. Theory Comput. 2012, 8, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, C.; Alkorta, I.; Elguero, J.; Sánchez-Sanz, G. Cooperative Effects in Weak Interactions: Enhancement of Tetrel Bonds by Intramolecular Hydrogen Bonds. Molecules 2019, 24, 308. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Competition between intramolecular hydrogen and pnictogen bonds in protonated systems. Theor. Chem. Accounts 2016, 135, 140. [Google Scholar] [CrossRef]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 084108. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Bannwarth, C.; Dohm, S.; Hansen, A.; Pisarek, J.; Pracht, P.; Seibert, J.; Neese, F. Fully automated quantum-chemistry-based computation of spin–spin-coupled nuclear magnetic resonance spectra. Angew. Chem. Int. Ed. 2017, 56, 14763–14769. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J. Chem. Phys. 2015, 143, 054107. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P.L.A. Quantum Chemical Topology: On Bonds and Potentials. In Intermolecular Forces and Clusters I. Structure and Bonding; Wales, D.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 115. [Google Scholar]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M.; Kaczmarek, A.; Sadlej, A.J. Estimates of the Energy of Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef]
- Muller, P. Glossary of Terms Used in Physical Organic Chemistry. Pure Appl. Chem. 1994, 66, 1077–1184. [Google Scholar] [CrossRef] [Green Version]
- McCleskey, T.M.; Ehler, D.S.; Keizer, T.S.; Asthagiri, D.N.; Pratt, L.R.; Michalczyk, R.; Scott, B.L. Beryllium displacement of H+ from strong hydrogen bonds. Angew. Chem. Int. Edit. 2007, 46, 2669–2671. [Google Scholar] [CrossRef] [PubMed]









| n | ∆HHB | ∆HInt |
|---|---|---|
| 0 | −8.7 | 1.8 |
| 1 | −16.8 | −0.6 |
| 2 | −24.6 | −0.5 |
| 3 | −16.1 | 0.1 |
| 4 | −16.6 | 0.1 |
| 5 | −17.5 | 0.3 |
| X | HOCHX(CH2)2CH2NH2 | HOCH2(CH2)2CHXNH2 |
|---|---|---|
| H | 0.038 | 0.038 |
| F | 0.046 | 0.032 |
| Cl | 0.053 | 0.031 |
| Br | 0.057 | 0.030 |
| n | O–H···N | N–H···O | N–H···O, O–H···F | Bridged |
|---|---|---|---|---|
| 0 | 22.2 | 13.2 | 8.9 | 0.0 |
| 1 | 23.4 | 30.8 | 19.9 | 0.0 |
| 2 | 9.7 | 32.0 | 24.7 | 0.0 |
| 3 | 8.7 | 33.4 | 27.2 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamsabhi, A.M.; Mó, O.; Yáñez, M. Perturbating Intramolecular Hydrogen Bonds through Substituent Effects or Non-Covalent Interactions. Molecules 2021, 26, 3556. https://doi.org/10.3390/molecules26123556
Lamsabhi AM, Mó O, Yáñez M. Perturbating Intramolecular Hydrogen Bonds through Substituent Effects or Non-Covalent Interactions. Molecules. 2021; 26(12):3556. https://doi.org/10.3390/molecules26123556
Chicago/Turabian StyleLamsabhi, Al Mokhtar, Otilia Mó, and Manuel Yáñez. 2021. "Perturbating Intramolecular Hydrogen Bonds through Substituent Effects or Non-Covalent Interactions" Molecules 26, no. 12: 3556. https://doi.org/10.3390/molecules26123556