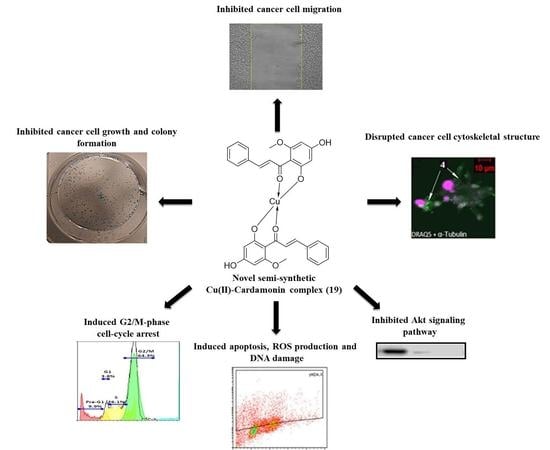
Novel Semi-Synthetic Cu (II)–Cardamonin Complex Exerts Potent Anticancer Activity against Triple-Negative Breast and Pancreatic Cancer Cells via Inhibition of the Akt Signaling Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Compound 19 Inhibited the Growth and Migration of MDA-MB-468 and PANC-1 Cancer Cells
2.2. Compound 19 Induced Gap 2 (G2)/Mitosis (M) Phase Cell-Cycle Arrest by Triggering DNA Damage in MDA-MB-468 and PANC-1 Cancer Cells
2.3. Compound 19 Causes Severe Disruption in Cytoskeletal Structure
2.4. Compound 19 Induced Caspase-Dependent Apoptosis and Reactive Oxygen Species (ROS) Generation in MDA-MB-468 and PANC-1 Cancer Cells
2.5. Compound 19 Exerted Its Cytotoxic Activity in MDA-MB-468 and PANC-1 Cells via Inhibition of Akt/4EBP1 Signalling and Downregulation of c-Myc
3. Materials and Methods
3.1. Synthesis of 19
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Colony Formation Assay
3.5. Migration Assay
3.6. Cell-Cycle Analysis
3.7. γ-H2AX Detection for DNA Damage Assessment
3.8. Confocal Microscopy
3.9. Annexin V-FITC/Propidium Iodide (PI) Apoptosis Assay
3.10. Caspase-Glo 3/7 Assay
3.11. Western Blotting Analysis
3.12. Reactive Oxygen Species (ROS) Detection
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 29 March 2020).
- Worldwide Cancer Incidence Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence (accessed on 29 March 2020).
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic Cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.V.; Carver, C.M.; Hastings, S.D.; Ramachandran, K.; Muniswamy, M.; Risinger, A.L.; Beutler, J.A.; Mooberry, S.L. Triple-negative breast cancer cell line sensitivity to englerin A identifies a new, targetable subtype. Breast Cancer Res. Treat. 2019, 177, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Break, M.K.B.; Chiang, M.; Wiart, C.; Chin, C.-F.; Khoo, A.S.B.; Khoo, T.-J. Cytotoxic activity of boesenbergia rotunda extracts against nasopharyngeal carcinoma cells (HK1). cardamonin, a boesenbergia rotunda constituent, inhibits growth and migration of HK1 cells by inducing caspase-dependent apoptosis and G2/M-phase arrest. Nutr. Cancer 2021, 73, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jeengar, M.K.; Thummuri, D.; Koval, A.; Katanaev, V.L.; Marepally, S.; Naidu, V.G.M. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. BioFactors 2017, 43, 152–169. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 7591. [Google Scholar] [CrossRef]
- Kong, W.; Li, C.; Qi, Q.; Shen, J.; Chang, K. Cardamonin induces G2/M arrest and apoptosis via activation of the JNK-FOXO3a pathway in breast cancer cells. Cell Biol. Int. 2020, 44, 177–188. [Google Scholar] [CrossRef]
- Break, M.K.B.; Hossan, M.S.; Khoo, Y.; Qazzaz, M.E.; Al-Hayali, M.Z.K.; Chow, S.C.; Wiart, C.; Bradshaw, T.D.; Collins, H.; Khoo, T.-J. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the MTOR pathway. Fitoterapia 2018, 125, 161–173. [Google Scholar] [CrossRef]
- Shahraki, S.; Saeidifar, M.; Delarami, H.S.; Kazemzadeh, H. Molecular docking and inhibitory effects of a novel cytotoxic agent with bovine liver catalase. J. Mol. Struct. 2020, 1205, 127590. [Google Scholar] [CrossRef]
- Mori, S.; Chang, J.T.; Andrechek, E.R.; Matsumura, N.; Baba, T.; Yao, G.; Kim, J.W.; Gatza, M.; Murphy, S.; Nevins, J.R. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene 2009, 28, 2796–2805. [Google Scholar] [CrossRef] [Green Version]
- Nandakumar, N.; Muthuraman, S.; Gopinath, P.; Nithya, P.; Gopas, J.; Kumar, R.S. Synthesis of coumaperine derivatives: Their NF-ΚB inhibitory effect, inhibition of cell migration and their cytotoxic activity. Eur. J. Med. Chem. 2017, 125, 1076–1087. [Google Scholar] [CrossRef]
- Wu, C.-F.; Efferth, T. Miltirone Induces G2/M cell cycle arrest and apoptosis in CCRF-CEM acute lymphoblastic leukemia cells. J. Nat. Prod. 2015, 78, 1339–1347. [Google Scholar] [CrossRef]
- Sone, K.; Piao, L.; Nakakido, M.; Ueda, K.; Jenuwein, T.; Nakamura, Y.; Hamamoto, R. Critical role of lysine 134 methylation on histone H2AX for γ-H2AX production and DNA repair. Nat. Commun. 2014, 5, 5691. [Google Scholar] [CrossRef] [Green Version]
- Hossan, M.S.; Chan, Z.-Y.; Collins, H.M.; Shipton, F.N.; Butler, M.S.; Rahmatullah, M.; Lee, J.B.; Gershkovich, P.; Kagan, L.; Khoo, T.-J.; et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/MTOR signal transduction inhibition. Cancer Lett. 2019, 453, 57–73. [Google Scholar] [CrossRef]
- Smedley, C.J.; Stanley, P.A.; Qazzaz, M.E.; Prota, A.E.; Olieric, N.; Collins, H.; Eastman, H.; Barrow, A.S.; Lim, K.-H.; Kam, T.-S.; et al. Sustainable syntheses of (−)-jerantinines A & E and structural characterisation of the jerantinine-tubulin complex at the colchicine binding site. Sci. Rep. 2018, 8, 10617. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Tang, Y.; An, R.; Lin, M.; Chen, L.; Du, J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017, 8, e3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.G.; Dias, C.; Matos, A.M.; Neng, N.; Nogueira, J.M.F.; Barreira, L.; Albericio, F.; et al. Isololiolide, a carotenoid metabolite isolated from the brown alga cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased P53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar] [CrossRef]
- Tsang, W.P.; Chau, S.P.Y.; Kong, S.K.; Fung, K.P.; Kwok, T.T. Reactive oxygen species mediate doxorubicin induced P53-independent apoptosis. Life Sci. 2003, 73, 2047–2058. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, Q.; Shi, D.; Niu, P.; Chen, Y.; Deng, J. MTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2014, 99, 44–51. [Google Scholar] [CrossRef]
- Niu, P.; Li, J.; Chen, H.; Zhu, Y.; Zhou, J.; Shi, D. Anti-proliferative effect of cardamonin on MTOR inhibitor-resistant cancer cells. Mol. Med. Rep. 2020, 21, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.-G.; Niu, P.-G.; Shi, D.-H.; Liu, Y.; Deng, J.; Chen, Y.-Y. Cardamonin inhibits angiogenesis by MTOR downregulation in SKOV3 cells. Planta Med. 2015, 82, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/MTOR pathway. Anticancer Drugs 2019, 30, 3733. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/MTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef]
- Wei, R.; Cortez Penso, N.E.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Epigallocatechin-3-gallate (EGCG) suppresses pancreatic cancer cell growth, invasion, and migration partly through the inhibition of Akt pathway and epithelial-mesenchymal transition: Enhanced efficacy when combined with gemcitabine. Nutrients 2019, 11, 1856. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.-H.; Wu, T.-Y.; Huang, Q.; Jin, G. New substituted quinoxalines inhibit triple-negative breast cancer by specifically downregulating the c-MYC transcription. Nucleic Acids Res. 2019, 47, 10529–10542. [Google Scholar] [CrossRef]
- Skoudy, A.; Hernández-Muñoz, I.; Navarro, P. Pancreatic ductal adenocarcinoma and transcription factors: Role of c-Myc. J. Gastrointest. Cancer 2011, 42, 76–84. [Google Scholar] [CrossRef]
- Buchholz, M.; Schatz, A.; Wagner, M.; Michl, P.; Linhart, T.; Adler, G.; Gress, T.M.; Ellenrieder, V. Overexpression of C-Myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006, 25, 3714–3724. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhou, X.; Xia, Q.; Shen, J.; Yan, J.; Zhu, J.; Li, X.; Shu, M. Long non-coding RNA CCAT1 that can be activated by c-Myc promotes pancreatic cancer cell proliferation and migration. Am. J. Transl. Res. 2016, 8, 5444–5454. [Google Scholar]
- Sodir, N.M.; Kortlever, R.M.; Barthet, V.J.A.; Campos, T.; Pellegrinet, L.; Kupczak, S.; Anastasiou, P.; Brown Swigart, L.; Soucek, L.; Arends, M.J.; et al. Myc instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov. 2020, 19, 435. [Google Scholar] [CrossRef] [Green Version]
- Qazzaz, M.E.; Raja, V.J.; Lim, K.-H.; Kam, T.-S.; Lee, J.B.; Gershkovich, P.; Bradshaw, T.D. In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine B. Cancer Lett. 2016, 370, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.M.; Abdelghany, M.K.; Messmer, M.; Yue, B.; Deeves, S.E.; Kindle, K.B.; Mantelingu, K.; Aslam, A.; Winkler, G.S.; Kundu, T.K.; et al. Differential effects of garcinol and curcumin on histone and P53 modifications in tumour cells. BMC Cancer 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Compound | GI50 (µM) 1 | Selectivity Index (SI) 2 | |||
|---|---|---|---|---|---|
| MDA-MB-468 | PANC-1 | MRC-5 | MDA-MB-468 | PANC-1 | |
| 19 | 6.14 ± 0.06 | 12.48 ± 0.70 | 32.60 ± 5.13 | 5.3 | 2.6 |
| Cardamonin | 34.33 ± 0.55 | 19.21 ± 2.37 | 43.25 ± 3.31 | 1.2 | 2.2 |
| Vincristine Sulphate | 0.03 ± 0.0005 | 0.48 ± 0.24 | 5.58 ± 0.38 | 186 | 11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossan, M.S.; Break, M.K.B.; Bradshaw, T.D.; Collins, H.M.; Wiart, C.; Khoo, T.-J.; Alafnan, A. Novel Semi-Synthetic Cu (II)–Cardamonin Complex Exerts Potent Anticancer Activity against Triple-Negative Breast and Pancreatic Cancer Cells via Inhibition of the Akt Signaling Pathway. Molecules 2021, 26, 2166. https://doi.org/10.3390/molecules26082166
Hossan MS, Break MKB, Bradshaw TD, Collins HM, Wiart C, Khoo T-J, Alafnan A. Novel Semi-Synthetic Cu (II)–Cardamonin Complex Exerts Potent Anticancer Activity against Triple-Negative Breast and Pancreatic Cancer Cells via Inhibition of the Akt Signaling Pathway. Molecules. 2021; 26(8):2166. https://doi.org/10.3390/molecules26082166
Chicago/Turabian StyleHossan, Md Shahadat, Mohammed Khaled Bin Break, Tracey D. Bradshaw, Hilary M. Collins, Christophe Wiart, Teng-Jin Khoo, and Ahmed Alafnan. 2021. "Novel Semi-Synthetic Cu (II)–Cardamonin Complex Exerts Potent Anticancer Activity against Triple-Negative Breast and Pancreatic Cancer Cells via Inhibition of the Akt Signaling Pathway" Molecules 26, no. 8: 2166. https://doi.org/10.3390/molecules26082166