Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils
Abstract
:1. Introduction
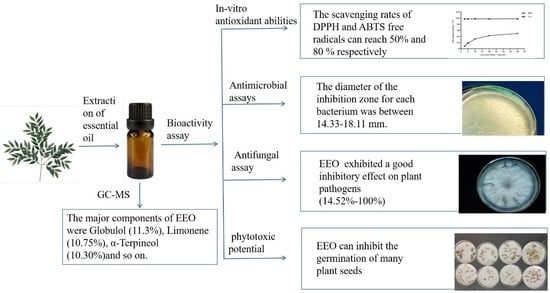
2. Results and Discussion
2.1. The Composition of EEO
2.2. The Antioxidant Abilities of EEO In Vitro
2.3. The Antibacterial Activity of EEO
2.4. The Antifungal Activity of EEO
2.5. The Phytotoxic Activity of EEO
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extraction of Essential Oil
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of EEO
3.4. In-Vitro Antioxidant Abilities
3.4.1. Determination of DPPH-Free Radical Scavenging Ability
3.4.2. Evaluation of ABTS-Free Radical Scavenging Ability
3.5. Antimicrobial Assays
3.5.1. Microbial Strains for Antimicrobial Screening
3.5.2. Determination of Inhibition Zones
3.5.3. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.5.4. Antifungal Assay
3.6. Phytotoxic Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Agrow. Agrow’s Top. 20: 2007 Edition-DS258; Informa Health Care: London, UK, 2007. [Google Scholar]
- Goldbeck, J.C.; Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; Silva, W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crops Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Khouja, M.L.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Safaei, G.J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharm. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Kohli, R.K. Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Z. Naturforsch. C J. Biosci. 2006, 61, 52–56. [Google Scholar] [CrossRef]
- He, H.; Song, Q.M.; Wang, Y.F.; Yu, S.X. Phytotoxic effects of volatile organic compounds in soil water taken from a Eucalyptus urophylla plantation. Plant Soil 2014, 377, 203–215. [Google Scholar] [CrossRef]
- Jouda, M.B.J.A.S.H.; Khouja, M.L. Efficacy of Eucalyptus essential oils fumigant control against Ectomyelois ceratoniae (lepidoptera: Pyralidae) under various space occupation conditions. J. Stored Prod. Res. 2013, 53, 67–71. [Google Scholar] [CrossRef]
- Afify, E.M.M.; Ali, F.S.; Turky, A.F. Control of Tetranychus urticae koch by extracts of three essential oils of chamomile, marjoram and Eucalyptus. Asian Pac. J. Trop. Biomed. 2012, 2, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi, K.M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial activity and chemical composition of 20 Eucalyptus species’ essential oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Lucia, A.; Gonzalez, A.P.; Seccacini, E.; Licastro, S.; Zerba, E.; Masuh, H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control. Assoc. 2007, 23, 299–303. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L.; Wang, E.I.C.; Chang, S.T. Antifungal activities and chemical compositions of essential oils from leaves of four Eucalyptus. Taiwan J. For. Sci. 2006, 21, 49–61. [Google Scholar] [CrossRef]
- Liu, X.X.; Chen, Q.B.; Wang, Z.H.; Xie, L.; Zhi, X. Phytotoxic effects of essential oil from Eucalyptus grandis × E. urophylla on pathogenic fungi and pest insects. Front. For. China 2008, 3, 232–236. [Google Scholar] [CrossRef]
- Zhou, L.J.; Huang, L.J.; Yang, Z.R.; Bai, L.H. Optimization of supercritical CO2 extraction conditions for essential oil from Eucalyptus grandis × Eucalyptus urophylla using box-behnken design-response surface methodology. J. Sichuan Univ. 2014, 51, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Keszei, A.; Brubaker, C.L.; Foley, W.J. A molecular perspective on terpene variation in Australian Myrtaceae. Aust. J. Bot. 2008, 56, 197. [Google Scholar] [CrossRef]
- Marzoug, H.N.B.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Romdhane, M. Eucalyptus (gracilis, oleosa, salubris, and salmonophloia) essential oils: Their chemical composition and antioxidant and antimicrobial activities. J. Med. Food 2010, 13, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Kaur, S.; Negi, K.; Kumari, S.; Saini, V.; Batish, D.R.; Kohli, R.K. Assessment of in vitro antioxidant activity of essential oil of Eucalyptus citriodora (lemon-scented Eucalyptus; Myrtaceae) and its major constituents. LWT Food Sci. Technol. 2012, 48, 237–241. [Google Scholar] [CrossRef]
- Hassine, D.B.; Abderrabba, M.; Yvon, Y.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Bouajila, J. Chemical Composition and in vitro evaluation of the antioxidant and antimicrobial activities of Eucalyptus gillii essential oil and extracts. Molecules 2012, 17, 9540–9558. [Google Scholar] [CrossRef] [Green Version]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Melusova, M.; Kozics, K.; Slamenova, D. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. J. Agric. Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef]
- Amiri, H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2012, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lado, C.; Then, M.; Varga, I.; Szoke, E.; Szentmihályi, K. Antioxidant property of volatile oils determined by the ferric reducing ability. Z. Naturforsch. C J. Biosci. 2004, 59, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Antonella, D.S.; Durazzi, F.; Sarpietro, M.G.; Mazzanti, G. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem. Toxicol. 2013, 60, 141–146. [Google Scholar] [CrossRef]
- Biljana, D.V.; Tatjana, Đ.; Danijela, Š. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from montenegro. Czech. J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [Green Version]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; Bruyne, T.D.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Barel, S.; Segal, R.; Yashphe, J. The antimicrobial activity of the essential oil from Achillea fragrantissima. J. Ethnopharmacol. 1991, 33, 187–191. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yang, Y.; Yu, H.D.; Yue, Y.; Zou, G. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar] [CrossRef]
- Sieniawska, E.; Los, R.; Baj, T.; Malm, A.; Glowniak, K. Antimicrobial efficacy of Mutellina purpurea essential oil and α-pinene against Staphylococcus epidermidis grown in planktonic and biofilm cultures. Ind. Crops Prod. 2013, 51, 152–157. [Google Scholar] [CrossRef]
- O’donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murbach, T.A.B.F.; Nunes, B.L.; Da, S.P.I.; Júnior, A.F. Antimicrobial activity of essential oils. J. Essent Oil. Res. 2013, 26, 34–40. [Google Scholar] [CrossRef]
- Freitas, P.R.; Carolina, A.; Araújo, J.D.; Rodrigues, C.; Coutinho, M. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crops Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Zhou, L.J.; Li, F.R.; Huang, L.J.; Yang, Z.R.; Yuan, S.; Bai, L.H. Antifungal activity of Eucalyptus oil against rice blast fungi and the possible mechanism of gene expression pattern. Molecules 2016, 21, 621. [Google Scholar] [CrossRef] [Green Version]
- Katooli, N.; Maghsodlo, R.; Razavi, S.E. Evaluation of Eucalyptus essential oil against some plant pathogenic fungi. J. Plant Breed. Crop Sci. 2011, 3, 41–43. Available online: http://www.academicjournals.org/jpbcs (accessed on 25 February 2021).
- España, M.D.; Arboleda, J.W.; Ribeiro, J.A.; Abdelnur, P.V.; Guzman, J.D. Eucalyptus leaf byproduct inhibits the anthracnose-causing fungus Colletotrichum gloeosporioides. Ind. Crops Prod. 2017, 108, 793–797. [Google Scholar] [CrossRef]
- Chang, H.T.; Cheng, Y.H.; Wu, C.L.; Chang, S.T.; Chang, T.T.; Su, Y.C. Antifungal activity of essential oil and its constituents from Calocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi. Bioresour. Technol. 2008, 99, 6266–6270. [Google Scholar] [CrossRef]
- An, P.P.; Yang, X.B.; Yu, J.; Qi, J.; Ren, X.; Kong, Q. α-terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Moreira, M.R.; Cruz, G.M.P.; Lopes, M.S.; Albuquerque, A.A.C.; Leal-Cardoso, J.H. Effects of terpineol on the compound action potential of the rat sciatic nerve. Braz J. Med. Biol. Res. 2001, 34, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultr astructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Aleu, J.; Hanson, J.R.; Galán, H.R.; Collado, I.G. Biotransformation of the fungistatic sesquiterpenoids patchoulol, ginsenol, cedrol and globulol by Botrytis cinerea. J. Mol. Catal. B Enzym. 2001, 11, 329–334. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus essential oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.X.; Wu, H.W.; Feng, Y.J.; Deng, S.Y.; Hou, A.J.; Che, F.F.; Liu, Y.Y.; Geng, Q.Q.; Ni, H.W.; Wei, Y. A strategy of rapidly screening out herbicidal chemicals from Eucalyptus essential oils. Pest Manag. Sci. 2019, 76, 917–927. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Liu, D.L.; Stanton, R. Chemical composition of essential oils of four Eucalyptus species and their phytotoxicity on silverleaf nightshade (Solanum elaeagnifolium Cav.) in Australia. Plant Growth Regul. 2012, 68, 231–237. [Google Scholar] [CrossRef]
- Abrahim, D.; Francischini, A.C.; Pergo, E.M.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol. Biochem. 2003, 41, 985–991. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, L.D.; Mancini, E.; Almeida, L.F.R.; Vincenzo, D.F. The antigerminative activity of twenty seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Nuria, P. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Ibrahim, M.; Zahiruddin, S.; Parveen, R.; Khan, W.; Ahmad, S.; Shrivastava, B.; Shrivastava, A.K. TLC-bioautography identification and GC-MS analysis of antimicrobial and antioxidant active compounds in Musa × paradisiaca L. fruit pulp essential oil. Phytochem. Anal. 2019, 30, 332–345. [Google Scholar] [CrossRef]
- Nikpour, H.; Mousavi, M.; Asadollahzadeh, H. Qualitative and quantitative analysis of Teucrium polium essential oil components by GC-MS coupled with MCR and PARAFAC methods. Phytochem. Anal. 2018, 29, 590–600. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]


| Number | Retention Time (min) | Retention Indices (RI) | Compounds | Percentage (%) |
|---|---|---|---|---|
| 1 | 9.564 | 931 | α-pinene | 17.02 |
| 2 | 9.906 | 943 | camphene | 2.83 |
| 3 | 11.466 | 1025 | o-cymenep-cymene | 5.76 |
| 4 | 11.605 | 1030 | eucalyptol | 1.35 |
| 5 | 11.703 | 1034 | trans-β-ocimene | 0.31 |
| 6 | 12.184 | 1047 | γ-terpinene | 0.51 |
| 7 | 13.677 | 1100 | fenchol | 4.68 |
| 8 | 14.032 | 1120 | α-campholenal | 2.25 |
| 9 | 15.088 | 1146 | isoborneol | 0.25 |
| 10 | 15.519 | 1148 | endo-borneol | 7.77 |
| 11 | 15.881 | 1161 | terpinen-4-ol | 2.04 |
| 12 | 16.548 | 1172 | α-terpineol | 13.63 |
| 13 | 17.646 | 1228 | Isobornyl formate | 1.37 |
| 14 | 18.01 | 1299 | myrtenyl acetate | 0.37 |
| 15 | 21.04 | 1373 | isoledene | 0.25 |
| 16 | 21.91 | 1424 | β-caryophyllene | 2.63 |
| 17 | 22.296 | 1439 | aromadendrene | 11.08 |
| 18 | 22.493 | 1456 | α-humulene | 0.36 |
| 19 | 22.872 | 1494 | γ-muurolene | 0.34 |
| 20 | 24.663 | 1530 | epiglobulol | 2.86 |
| 21 | 25.77 | 1597 | ledol | 0.65 |
| 22 | 26.755 | 1628 | τ-cadinol | 0.54 |
| 23 | 27.188 | 1641 | α-cadinol | 0.56 |
| Parameters | E. coli (−) | B. subtilis (+) | P. aeruginosa (−) | S. aureus (+) | S. typhimurium (−) | B. cereus (+) |
|---|---|---|---|---|---|---|
| Inhibition zone (mm) | 15.22 ± 0.38 | 14.33 ± 0.33 | 15.17 ± 0.24 | 15.11 ± 0.84 | 18.11 ± 0.19 | 15.78 ± 0.38 |
| MIC (mg/mL) | 0.091 | 0.091 | 0.023 | 0.045 | 0.023 | 0.045 |
| MBC (mg/mL) | 10 | 10 | 10 | 10 | 10 | 10 |
| Inhibition Rate (%) | The Concentration of EEO (mg/mL) | |||||
|---|---|---|---|---|---|---|
| Strains | 2.5 | 5 | 10 | 20 | 40 | |
| Trichoderma longibrachiatum | 15.96 ± 2.42 | 47.15 ± 5.67 | 90.91 ± 5.82 | 100 | 100 | |
| Botrytis cinerea | 16.74 ± 1.98 | 68.44 ± 1.71 | 100 | 100 | 100 | |
| Colletotrichum acutatum | 14.44 ± 2.77 | 40.45 ± 2.06 | 78.58 ± 1.30 | 100 | 100 | |
| Colletotrichum gloeosporioides | 41.29 ± 1.91 | 82.57 ± 4.08 | 100 | 100 | 100 | |
| Fusarium oxyspoyum | 34.95 ± 2.20 | 44.46 ± 1.62 | 78.38 ± 1.40 | 100 | 100 | |
| Fusarium graminearum | 16.97 ± 3.85 | 32.88 ± 2.33 | 45.49 ± 1.01 | 61.52 ± 1.84 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils. Molecules 2021, 26, 1450. https://doi.org/10.3390/molecules26051450
Zhou L, Li J, Kong Q, Luo S, Wang J, Feng S, Yuan M, Chen T, Yuan S, Ding C. Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils. Molecules. 2021; 26(5):1450. https://doi.org/10.3390/molecules26051450
Chicago/Turabian StyleZhou, Lijun, Jiajia Li, Qingbo Kong, Siyuan Luo, Jie Wang, Shiling Feng, Ming Yuan, Tao Chen, Shu Yuan, and Chunbang Ding. 2021. "Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils" Molecules 26, no. 5: 1450. https://doi.org/10.3390/molecules26051450