Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of the Pristine and Nano-Magnetite Modified Biochar
2.2. Adsorption Performances and Kinetics
2.3. Adsorption Isotherm
2.4. Effect of pH and Ionic Strength on Cr (VI) Adsorption
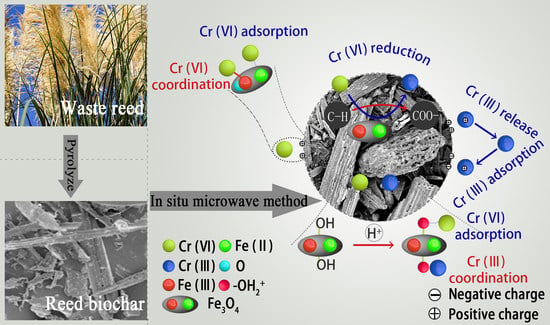
2.5. Cr Adsorption Mechanism by the Nano-Magnetite-Modified Biochar (m-Biochar)
3. Materials and Methods
3.1. Biochar Materials, Groundwater and Chemicals
3.2. Nano-Magnetite Modified Biochar: Fabrication and Characterisation
3.3. Batch Experiments
3.4. Calculations
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, T.; Hu, L.; Zhang, M.; Jiang, M.; Fiedler, H.; Bai, W.; Wang, X.; Zhang, D.; Li, Z. Cr(VI) removal from soils and groundwater using an integrated adsorption and microbial fuel cell (A-MFC) technology. Environ. Pollut. 2019, 252, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Pittman Jr, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanopa-rticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, C.; Wei, W.; Ni, B. Magnetic poly (aniline-co-5-sulfo-2-anisidine) as multifunctional adsorbent for highly effective co-removal of aqueous Cr(VI) and 2,4-Dichlophenol. Chem. Eng. J. 2020, 387, 124152. [Google Scholar] [CrossRef]
- Shanker, A.K. Chromium: Environmental contamination, Health Effects and Mode of Action. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 624–633. [Google Scholar]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Zhang, N.; Fang, Z.; Zhang, R. Comparison of several amendments forin-site remediating chromium-contaminated farmland soil. Water Air Soil Pollut. 2017, 228, 1–10. [Google Scholar] [CrossRef]
- Jiang, B.; Gong, Y.; Gao, J.; Sun, T.; Liu, Y.; Oturan, N.; Oturan, M. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: State-of-the-art and perspectives. J. Hazard. Mater. 2019, 365, 205–226. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Li, M.; Liu, X. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): Effects of materials in different status. Sci. Total Environ. 2020, 717, 137112. [Google Scholar] [CrossRef] [PubMed]
- Prevot, A.; Ginepro, M.; Peracaciolo, E.; Zelano, V.; De Luca, D. Chemical vs bio-mediated reduction of hexavalent chromium. An in-vitro study for soil and deep waters remediation. Geoderma 2018, 312, 17–23. [Google Scholar] [CrossRef]
- Liu, C.; Fiol, N.; Villaescusa, I.; Poch, J. New approach in modeling Cr(VI) sorption onto biomass from metal binary mixtures solutions. Sci. Total Environ. 2016, 541, 101–108. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xiang, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Yu, Y.; An, Q.; Jin, L.; Luo, N.; Li, Z.; Jiang, J. Unraveling sorption of Cr (VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: Implicit mechanism and application. Bioresour. Technol. 2020, 297, 122466. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Zhang, Y.; Wang, L.; Chen, J.; Jiang, Y.; Tsang, D.; Zhao, Z.; Ren, S.; Liu, Z.; Crittenden, J. Mechanistic insights into adsorption and reduction of hexavalent chromium from water using magnetic biochar composite: Key roles of Fe3O4 and persistent free radicals. Environ. Pollut. 2018, 243, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.; Wang, D.; Lin, H.; Huang, L. Removal and reduction of Cr(VI) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge. J. Clean Prod. 2020, 257, 120562. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman Jr, C.U.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Liyanage, A.; Canaday, S.; Pittman, C., Jr.; Mlsna, T. Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents. Chemosphere 2020, 258, 127336. [Google Scholar] [CrossRef]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Key role of FeO in the reduction of Cr(VI) by magnetic biochar synthesised using steel pickling waste liquor and sugarcane bagasse. J. Clean Prod. 2020, 245, 118886. [Google Scholar] [CrossRef]
- Liu, Y.; Sohi, S.; Liu, S.; Guan, J.; Zhou, J.; Chen, J. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 2019, 235, 276–281. [Google Scholar] [CrossRef]
- Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(VI) by a mild one-step hydrothermal method from peanut hull. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 563, 102–111. [Google Scholar] [CrossRef]
- Karunanayake, A.; Todd, O.; Crowley, M.; Ricchetti, L.; Pittman, C., Jr.; Anderson, R.; Mlsna, T. Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir. Chem. Eng. J. 2017, 319, 75–88. [Google Scholar] [CrossRef]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull onremoval of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Ma, S.; Zhu, B.; Zhang, J.; Zheng, C. Mercury Removal by Magnetic Biochar Derived from Simultaneous Activation and Magnetization of Sawdust. Environ. Sci. Technol. 2016, 50, 12040–12047. [Google Scholar] [CrossRef] [PubMed]
- Bielská, L.; Škulcová, L.; Neuwirthová, N.; Cornelissen, G.; Hale, S. Sorption, bioavailability and ecotoxic effects of hydrophobic organic compounds in biochar amended soils. Sci. Total. Environ. 2018, 624, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Zhu, J.; Muhammad, N.; Sheng, T.; Xu, X. Effect of synthesis methods on magnetic Kans grass biochar for enhanced As(III,V) adsorption from aqueous solutions. Biomass Bioenergy 2014, 71, 299–310. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Su, J.; Lyu, T.; Yi, H.; Bi, L.; Pan, G. Superior arsenate adsorption and comprehensive investigation of adsorption mechanism on novel Mn-doped La2O2CO3 composites. Chem. Eng. J. 2020, 391, 123623. [Google Scholar] [CrossRef]
- Fan, H.; Ma, X.; Zhou, S.; Huang, J.; Liu, Y.; Liu, Y. Highly efficient removal of heavy metal ions by carboxymethyl cellulose-immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef]
- Tytlak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr (VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Mao, S.; Chen, H.; Huang, L.; Qiu, R. Pb(II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour. Technol. 2013, 147, 545–552. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef]
- Su, J.; Bi, L.; Wang, C.; Lyu, T.; Pan, G. Enhancement of cadmium removal by oxygen-doped carbon nitride with molybdenum and sulphur hybridization. J. Colloid Interface Sci. 2019, 556, 606–615. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, C.; Guan, Q.; Ning, P.; Gu, J.; Chen, Q.; Zhang, J.; Miao, R. Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 2018, 195, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zheng, S.; Yang, L. Magnetic zirconium-based metal-organic frameworks for selective phosphate adsorption from water. J. Colloid Interface Sci. 2019, 552, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jiang, Y.; Yang, J.; Lin, D. Correlations and adsorption mechanisms of aromatic compounds on biochars produced from various biomass at 700 °C. Environ. Pollut. 2018, 233, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef]
- Ziaeifar, N.; Khosravi, M.; Behnajady, M.; Sohrabi, M.; Modirshahla, N. Optimizing adsorption of Cr(VI) from aqueous solutions by NiO nanoparticles using Taguchi and response surface methods. Water Sci. Technol. 2015, 72, 721–729. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Yousaf, B.; Biao, F.; Ullah, H.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Liu, R. Simultaneous functionalization and magnetization of biochar via NH3 ambiance pyrolysis for efficient removal of Cr (VI). Chemosphere 2018, 208, 712–721. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Chen, Y.; Li, Y. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour. Technol. 2020, 315, 123834. [Google Scholar] [CrossRef]
- Qin, J.; Li, Q.; Liu, Y.; Niu, A.; Lin, C. Biochar-driven reduction of As(V) and Cr(VI): Effects of pyrolysis temperature and low-molecular-weight organic acids. Ecotox. Environ. Saf. 2020, 201, 110873. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, K.; Singh, V. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J. Hazard. Mater. 2006, 135, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Shang, X.; Zhang, B.; Zhang, W.; Su, A.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.; Chen, M. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef]
- Pham, T.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of anionic azo dye onto large α-alumina beads. Colloid Polym. Sci. 2015, 293, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Wang, J.; Li, R.; Park, J.; Meng, Y.; Zhou, B.; Pensky, S.; Zhang, Z. Enhanced sorption of hexavalent chromium [Cr(VI)] from aqueous solutions by diluted sulfuric acid-assisted MgO-coated biochar composite. Chemosphere 2018, 208, 408–416. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, M.; Tang, T.; Xing, Q.; Peng, C.; Li, F.; Liu, H.; Luo, X.; Zou, J.; Min, X.; et al. Mechanism investigation of anoxic Cr(VI) removal by nano zero-valent iron based on XPS analysis in time scale. Chem. Eng. J. 2018, 335, 945–953. [Google Scholar] [CrossRef]
- Mokadem, Z.; Saidi-Besdes, S.; Lebaz, N.; Elaissari, A. Magnetic monolithic polymers prepared from high internal phase emulsions and Fe3O4 triazole-functionalized nanoparticles for Pb2+, Cu2+ and Zn2+ removal. React. Funct. Polym. 2020, 155, 104693. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; He, C. Removal of Cr(VI) and Hg(II) ions from wastewater by novel β-CD/MGO-SO3H composite. Colloids Surf. A 2017, 512, 129–136. [Google Scholar] [CrossRef]
- Song, X.M.; Wen, Y.J.; Wang, Y.Y.; Adeel, M.; Yang, Y. Environmental risk assessment of the emerging EDCs contaminants from rural soil and aqueous sources: Analytical and modelling approaches. Chemosphere 2018, 198, 546–555. [Google Scholar] [CrossRef]






| Materials | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|
| biochar | 51.23 | 0.02 | 0.01 |
| m-biochar | 154.79 | 0.09 | 0.05 |
| Cr (VI) (mg/L) | Materials | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | Intra-Particle Diffusion Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (h−1) | R2 | qe (mg/g) | k1 [g/(mg·h)] | R2 | kd1 [mg/(g·h0.5)] | C1 | R12 | kd2 [mg/(g·h0.5)] | C2 | R22 | ||
| 20 | biochar | 5.40 | 0.08 | 0.86 | 5.55 | 0.04 | 0.92 | 0.56 | 1.34 | 1.00 | 0.12 | 4.38 | 0.66 |
| m-biochar | 5.83 | 0.44 | 0.86 | 6.22 | 0.08 | 0.92 | 0.66 | 1.97 | 1.00 | 0.13 | 5.19 | 0.73 | |
| 40 | biochar | 5.60 | 0.45 | 0.85 | 6.02 | 0.08 | 0.92 | 0.63 | 1.79 | 0.94 | 0.13 | 5.04 | 0.49 |
| m-biochar | 6.47 | 0.59 | 0.88 | 6.74 | 0.12 | 0.94 | 0.72 | 2.52 | 0.97 | 0.06 | 6.41 | 0.80 | |
| 80 | biochar | 6.38 | 0.32 | 0.92 | 6.94 | 0.05 | 0.97 | 1.00 | 1.05 | 0.85 | 0.12 | 5.82 | 0.62 |
| m-biochar | 7.16 | 0.63 | 0.86 | 7.46 | 0.12 | 0.92 | 0.70 | 3.05 | 0.96 | 0.12 | 6.74 | 0.75 | |
| Materials | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | kL (L/mg) | R2 | n | kf (μg/g)/(μg/L)n | R2 | |
| biochar | 6.81 | 0.86 | 0.90 | 0.13 | 4.07 | 0.98 |
| m-biochar | 7.72 | 0.77 | 0.95 | 0.14 | 4.48 | 0.92 |
| pH | Adsorbents | Zeta Potential | Reference |
|---|---|---|---|
| 2 | Iron/zinc Biochar(Fe@Zn@HBC) | 42.9 | [12] |
| 2 | ZVI Magnetic Biochar(FeBC800) | ~23 | [18] |
| 2–4 | MPHC-HDA | >0 | [19] |
| 3 | Palm fiber biochar(BC) | ~5 | [39] |
| 3 | MBCO a | ~15 | [39] |
| Below 4.2 | N-doped magnetic agar biochar (ABF-N800) | >0 | [40] |
| 4.61 | Magnetic Biochar (SMBC2) | 45.7 | [17] |
| 7–11 | NiAl layered double oxides modified magnetic corncob biochar | <0 | [41] |
| 10.11 | Pennisetum hydridum biochar | −48.1 | [42] |
| 3 | biochar | 7.99 | This paper |
| 5 | biochar | 3.72 | This paper |
| 7–11 | biochar | <0 | This paper |
| 3 | m-biochar | 26.7 | This paper |
| 5 | m-biochar | 19.7 | This paper |
| 7–11 | m-biochar | <0 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhang, Y.; Cao, N.; Sun, D.; Zhang, Z.; Wang, Y.; Wen, Y.; Yang, Y.; Lyu, T. Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis. Molecules 2021, 26, 103. https://doi.org/10.3390/molecules26010103
Song X, Zhang Y, Cao N, Sun D, Zhang Z, Wang Y, Wen Y, Yang Y, Lyu T. Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis. Molecules. 2021; 26(1):103. https://doi.org/10.3390/molecules26010103
Chicago/Turabian StyleSong, Xiaoming, Yuewen Zhang, Nan Cao, Dong Sun, Zhipeng Zhang, Yunlong Wang, Yujuan Wen, Yuesuo Yang, and Tao Lyu. 2021. "Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis" Molecules 26, no. 1: 103. https://doi.org/10.3390/molecules26010103