Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix
Abstract
:1. Introduction
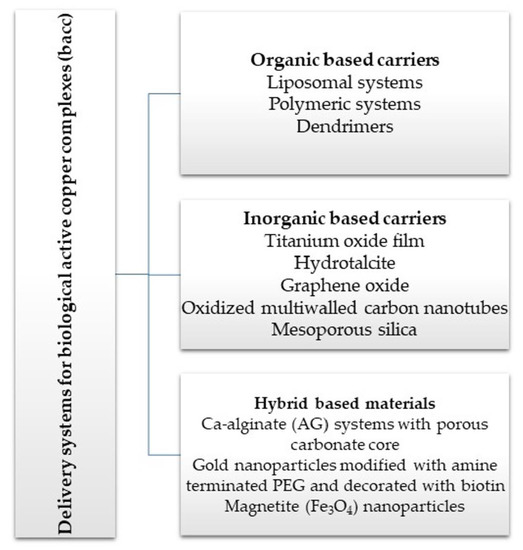
2. Delivery Systems for Copper Active Complexes
2.1. Organic-Based Carriers
2.1.1. Liposomal Systems
2.1.2. Polymeric Systems
2.1.3. Dendrimers
2.2. Inorganic Based Carriers
2.3. Hybrid-Based Materials
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AG | alginate |
| ALB | albumin |
| AngII | angiotensin II |
| bacc | biologically active copper complexes |
| BBB | blood brain barrier |
| bFGF | basic fibroblast growth factor |
| BPEI | branched polyethyleneimine |
| bpy | 2,2′-bipyridine |
| BSA | bovine serum albumin |
| CD | Cyclodextrins |
| CHEMS | cholesteryl hemisuccinate |
| CHLN | choline |
| CHOL | cholesterol |
| CMC | carboxymethyl cellulose |
| CNC | cellulose nanocrystals |
| COX2 | cyclooxgenase-2 |
| CPC | cetylpyridinium chloride |
| CS | chitosan |
| CT DNA | calf thymus DNA |
| dien | diethylenetriamine |
| dsf | disulfiram |
| DMβCD | heptakis-2,6-O-dimethyl-β-cyclodextrin |
| DMPC | dimyristoyl phosphatidylcholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine |
| DPPE-PEG2000 | dipalmitoylphosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] ammonium salt |
| dpphen | 4,7-diphenyl-1,10-phenanthroline |
| dppz | dipyrido[3,2-a:2′,3′-c]phenazine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DSPE | distearoyl phosphatidylethanolamine |
| EPR effect | Enhanced Permeability and Retention effect |
| HA | Hyaluronic acid |
| Hacac | acetylacetone |
| H2atsm | diacetylbis(N-4-methylthiosemicarbazone |
| H3btc | benzene tricarboxylic acid |
| Hchz | 6-hydroxychromone-3-carbaldehyde-(3′-hydroxy)benzoylhydrazone |
| Hddc | diethyldithiocarbamic acid |
| HGly | glycine |
| HGlu | gluconic acid |
| HHis | histidine |
| Him | indomethacin |
| HIm | imidazole |
| HP-β-CD | hydroxypropyl β cyclodextrin |
| H2ppt | polymerized-p-phenylenediamine-5,10,15,20-tetra-(4-aminophenyl)porphyrin |
| HSPC | hydrogenated soybean phosphatidylcholine |
| ICC | Indian childhood cirrhosis |
| inh | isoniazid |
| IP | iminopyridine |
| i.v. | intravenous |
| L-phe | L-phenylalanine |
| L-Thr | L-threonine |
| MCAO | middle cerebral artery occlusion |
| MIC | minimal inhibitory concentration |
| MRSA | methicillin-resistant S. aureus |
| MS | microspheres |
| MSS | mesoporous silica |
| MwCNTs | multiwalled carbon nanotubes |
| NAD | nicotinammide adenine dinucleotide |
| ncr | neocuproine |
| NDPMA | N-[3-(dimethylamino)-propyl]methacrylamide |
| NPCCs | neonatal pancreatic porcine cell clusters |
| NPs | nanoparticles |
| OPDMA | poly[2-(N-oxide-N,N-dimethylamino)ethyl methacrylate] |
| PAMAM | polyamidoamine |
| PC | phosphatidylcholine |
| pcc | polymer copper(II) complex |
| PECAM | platelet endothelial cell adhesion molecule |
| PEI | polyethyleneimine |
| PEIePEG | poly(ethyleneimine)epoly(ethyleneglycol) |
| PEG | poly(ethylene glycol) |
| PEG-DET | PEG-b-poly(aspartate diethyltriamine) |
| PEG-PBD | poly(ethylene glycol)-polybutadiene |
| PEGPLGA | methoxy poly(ethylene glycol)-b-poly(d,l-lactic-co-glycolic) acid |
| PEG-pLL50 | methoxy-poly(ethylene glycol)-block-poly(l-lysine hydrochloride) |
| PEG-PLL | poly(ethylene glycol)-b-poly(l-lysine) |
| PEG-PPO-PEG | poly(ethylene glycol)-block-poly(propylene oxide)-block-poly(ethylene glycol) |
| PG | phosphatidyl glycerol |
| phen | 1,10-phenantroline |
| PLA | poly(d,l-lactide) |
| PLGA | poly(d,l-lactide co-glycolide) |
| Plvap | plasmalemmal vesicle-associated protein |
| PPCN | poly-(polyethyleneglycol citrate-co-N-isopropylacrylamide) |
| PVC | polyvinyl chloride |
| ROS | reactive oxygen species |
| SCC | sodium copper chlorophyllin |
| SLT | soybean lecithin |
| SOD | superoxide dismutase |
| SA | stearylamine |
| SAG | Sodium alginate |
| TNF | tumor necrosis factor α |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
References
- Dollwet, H.H.A.; Sorenson, J.R.J. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Ogata, R.; Chong, P.F.; Maeda, K.; Imagi, T.; Nakamura, R.; Kawamura, N.; Kira, R. Long surviving classical Menkes disease treated with weekly intravenous copper therapy. J. Trace Elem. Med. Biol. 2019, 54, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Bryszewska, M.A. Comparison Study of Iron Bioaccessibility from Dietary Supplements and Microencapsulated Preparations. Nutrients 2019, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef]
- Park, S.J.; Park, Y.M. Eco-dyeing and antimicrobial properties of chlorophyllin copper complex extracted from Sasa veitchii fiber. Polymer 2010, 11, 357–362. [Google Scholar]
- Zhang, J.; Wang, W.; Yang, F.; Zhou, X.; Jin, H.; Yang, P.-Y. Comparative proteomic analysis of drug sodium iron chlorophyllin addition to Hep 3B cell line. Analyst 2012, 137, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hoshi, K.; Yanagawa, A.; Takano, K. Topical application of superoxide dismutase cream. Drugs Exp. Clin. Res. 1991, 17, 127–131. [Google Scholar]
- Churilova, I.V.; Zinov’ev, E.V.; Paramonov, B.A.; Drozdova, Y.I.; Sidel’nikov, V.O.; Chebotarev, V.Y. Effect of Erysod (erythrocyte superoxide dismutase) on blood concentration of reactive oxygen species in patients with severe burns and burn shock. Bull. Exp. Biol. Med. 2002, 134, 454–456. [Google Scholar] [CrossRef]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef]
- Carillon, J.; Rouanet, J.-M.; Cristol, J.-P.; Brion, R. Superoxide Dismutase Administration, A Potential Therapy Against Oxidative Stress Related Diseases: Several Routes of Supplementation and Proposal of an Original Mechanism of Action. Pharm. Res. 2013, 30, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, A.K.; Shakeel, A.; Kumar, V.; Singh, A.; Gupta, A.; Suhag, D.; Rajput, S.K.; Mukherjee, M. Therapeutic implications of superoxide dismutase and its importance in kinase drug discovery. Curr. Top. Med. Chem. 2017, 17, 2495–2508. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, J.R. Cu, Fe, Mn, and Zn Chelates Offer a Medicinal Chemistry Approach to Overcoming Radiation Injury. Curr. Med. Chem. 2002, 9, 639–662. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.H.; Beckman, J.S.; Shaw, C.A. Neuroprotective effect of CuATSM on neurotoxin-induced motor neuron loss in an ALS mouse model. Neurobiol. Dis. 2019, 130, 104495. [Google Scholar] [CrossRef]
- Gracia-Mora, I.; Ruiz-Ramirez, L.; G6mez-Ruiz, C.; Tinoco-Mndez, M.; Mtirquez-Quifiones, A.; Romero-De Lira, L.; Marin-Hernandez, A.; Madas-Rosales, L.; Bravo-Gdmez, M.E. Knigth’s move in the periodic table, from copper to platinum, novel antitumor mixed chelate copper compounds, casiopeinas, evaluated by an in vitro human and murine cancer cell line panel. Metal Based Drugs 2001, 43, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chaudhary, R.; Cohen, A.L.; Fink, K.; Goldlust, S.; Boockvar, J.; Chinnaiyan, P.; Wan, L.; Marcus, S.; Campian, J.L. A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma. J. Neuro-Oncol. 2019, 142, 537–544. [Google Scholar] [CrossRef]
- Wang, T.; Gu, Z. Copper in Medicine: Homeostasis, Chelation Therapy and Antitumor Drug Design. Curr. Med. Chem. 2006, 13, 525–537. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in Diseases and Treatments, and Copper-Based Anticancer Strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many ‘‘faces’’ of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Barile, A.; Arciello, M.; Rossi, L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J. Trace Elem. Med. Biol. 2019, 55, 204–213. [Google Scholar] [CrossRef]
- Quist, D.A.; Diaz, D.E.; Liu, J.J.; Karlin, K.D. Activation of dioxygen by copper metalloproteins and insights from model complexes. J. Biol. Inorg. Chem. 2017, 22, 253–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehbe, M.; Leung, Y.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective–Can copper complexes be developed as a novel class of therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Murillo, R.; Garcia-Ramos, J.C.; Ruiz-Azuara, L.; Cheatham, T.E., III; Cortes-Guzman, F. Intercalation processes of copper complexes in DNA. Nucleic Acids Res. 2015, 43, 5364–5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingle, A.P.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; da Silva Martins, L.H.; Rai, M. Chapter 4 Copper in medicine: Perspectives and toxicity. In Biomedical Applications of Metals; Rai, M., Ingle, A.P., Medici, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 95–112. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clinic. Toxicol. 2011, S3. [Google Scholar] [CrossRef] [Green Version]
- Oon, S.; Yap, C.-H.; Ihle, B.U. Acute copper toxicity following copper glycinate injection. Intern. Med. J. 2006, 36, 741–743. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2020, 12, 476. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper Complexes in Cancer Therapy. Met. Ions Life Sci. 2018, 18, 469–506. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg. Med. Chem. Lett. 2020, 30, 126905. [Google Scholar] [CrossRef] [PubMed]
- Shagufta, A.I. Transition metal complexes as proteasome inhibitors for cancer treatment. Inorg. Chim. Acta 2020, 506, 119521. [Google Scholar] [CrossRef]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Frączek, T.; Markowicz, M.; Mikiciuk-Olasik, E. Development of copper based drugs, radiopharmaceuticals and medical materials. Biometals 2012, 25, 1089–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostas, A.M.; Badea, M.; Ruţă, L.L.; Farcaşanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Čelan Korošin, N.; Cerc Korošec, R.; et al. Copper(II) complexes with mixed heterocycle ligands as promising antibacterial and antitumor species. Molecules 2020, 25, 3777. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Chylewska, A.; Biedulska, M.; Sumczyński, P.; Makowski, M. Metallopharmaceuticals in therapy—A new horizon for scientific research. Curr. Med. Chem. 2017, 25, 1729–1791. [Google Scholar] [CrossRef]
- Shobha Devi, C.; Thulasiram, B.; Aerva, R.R.; Nagababu, P. Recent Advances in Copper Intercalators as Anticancer Agents. J. Fluoresc. 2018, 28, 1195–1205. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of copper complexes with nucleic acids, Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem. Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Maeda, H. Polymer therapeutics and the EPR effect. J. Drug Target. 2017, 25, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Callari, M.; Aldrich-Wright, J.R.; de Souza, P.L.; Stenzel, M.H. Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment. Progress Polym. Sci. 2014, 39, 1614–1643. [Google Scholar] [CrossRef]
- Poursharifi, M.; Wlodarczyk, M.T.; Mieszawska, A.J. Nano-Based Systems and Biomacromolecules as Carriers for Metallodrugs in Anticancer Therapy. Inorganics 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [Green Version]
- Pinho, J.O.; Amaral, J.D.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 2019, 14, 835–850. [Google Scholar] [CrossRef]
- da Silva, P.B.; de Souza, P.C.; Fioramonti Calixto, G.M.; de O’Lopes, E.; Frem, R.C.G.; Netto, A.V.G.; Mauro, A.E.; Pavan, F.R.; Chorilli, M. In Vitro Activity of Copper(II) Complexes, Loaded or Unloaded into a Nanostructured Lipid System, against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2016, 17, 745. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.B.; Bonifacio, B.V.; Frem, R.C.G.; Godoy Netto, A.V.; Mauro, A.E.; Da Costa Ferreira, A.M.; Lopes, E.O.; Raddi, M.S.G.; Bauab, T.M.; Pavan, F.R.; et al. A nanostructured lipid system as a strategy to improve the in vitro antibacterial activity of copper(II) complexes. Molecules 2015, 20, 22534–22545. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Backstrom, I.; Leung, A.; Chen, K.; Malhotra, A.; Edwards, K.; Bally, M.B. Nanoscale Reaction Vessels Designed for Synthesis of Copper-Drug Complexes Suitable for Preclinical Development. PLoS ONE 2016, 11, e0153416. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zeng, S.; Lin, T.-M.; Krugner-Higby, L.; Lyman, D.; Steffen, D.; Xiong, M.P. Evaluating the anticancer properties of liposomal copper in a nude xenograft mouse model of human prostate cancer: Formulation, in vitro, in vivo, histology and tissue distribution studies. Pharm. Res. 2014, 31, 3106–3119. [Google Scholar] [CrossRef] [Green Version]
- Gaál, A.; Garay, T.M.; Horváth, I.; Máthé, D.; Szöllosi, D.; Veres, D.S.; Mbuotidem, J.; Kovács, T.; Tóvári, J.; Bergmann, R.; et al. Development and In Vivo Application of a Water-Soluble Anticancer Copper Ionophore System Using a Temperature-Sensitive Liposome Formulation. Pharmaceutics 2020, 12, 466. [Google Scholar] [CrossRef]
- Chen, K.T.J.; Anantha, M.; Leung, A.W.Y.; Kulkarni, J.A.; Militao, G.G.C.; Wehbe, M.; Sutherland, B.; Cullis, P.R.; Bally, M.B. Characterization of a liposomal copper(II)-quercetin formulation suitable for parenteral use. Drug Deliv. Transl. Res. 2020, 10, 202–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, B.; Kaur, G.; Chaudhary, G.R.; Sharma, V.K.; Srinivasan, H.; Mitra, S.; Sharma, A.; Gawali, S.L.; Hassan, P.A. An investigation of morphological, microscopic dynamics, fluidity, and physicochemical variations in Cu-decorated metallosomes with cholesterol. J. Mol. Liq. 2020, 318, 114034. [Google Scholar] [CrossRef]
- Vorauer-Uhl, K.; Fürnschlief, E.; Wagner, A.; Ferko, B.; Katinger, H. Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. Eur. J. Pharm. Sci. 2001, 14, 63–67. [Google Scholar] [CrossRef]
- Corvo, L.M.; Jorge, J.C.; Van’t Hof, R.; Cruz, M.E.; Crommelin, D.J.; Storm, G. Superoxide dismutase entrapped in long-circulating liposomes: Formulation design and therapeutic activity in rat adjuvant arthritis. Biochim. Biophys. Acta 2002, 1564, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Corvo, L.M.; Marinho, H.S.; Marcelino, P.; Lopes, R.M.; Vale, C.A.; Marques, C.R.; Martins, L.C.D.; Laverman, P.; Storm, G.; Martins, M.B.A.F. Superoxide dismutase enzymosomes: Carrier capacity optimization, in vivo behaviour and therapeutic activity. Pharm. Res. 2015, 32, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Galović Rengel, R.; Barisić, K.; Pavelić, Z.; Zanić Grubisić, T.; Cepelak, I.; Filipović Grcić, J. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur. J. Pharm. Sci. 2002, 15, 441–448. [Google Scholar] [CrossRef]
- Ishihara, T.; Nara, S.; Mizushima, T. Interactions of lecithinized superoxide dismutase with serum proteins and cells. J. Pharm. Sci. 2014, 103, 1987–1994. [Google Scholar] [CrossRef]
- Veronese, F.M.; Caliceti, P.; Schiavon, O.; Sergi, M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002, 54, 587–606. [Google Scholar] [CrossRef]
- Reddy, M.K.; Wu, L.; Kou, W.; Ghorpade, A.; Labhasetwar, V. Superoxide dismutase loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl. Biochem. Biotechnol. 2008, 151, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.K.; Labhasetwar, V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: An effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009, 23, 1384–1395. [Google Scholar] [CrossRef]
- Manickam, D.S.; Brynskikh, A.M.; Kopanic, J.L.; Sorgen, P.L.; Klyachko, N.L.; Batrakova, E.V.; Bronich, T.K.; Kabanov, A.V. Well-defined cross-linked antioxidant nanozymes for treatment of ischemic brain injury. J. Control. Release 2012, 162, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaugh, E.G.; Roat, J.W.; Gao, L.; Yang, R.-F.; Manickam, D.S.; Yin, J.X.; Schultz, H.D.; Bronich, T.K.; Batrakova, E.V.; Kabanov, A.V.; et al. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 2010, 31, 5218–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovagnoli, S.; Luca, G.; Casaburi, I.; Blasi, P.; Macchiarulo, G.; Ricci, M.; Calvitti, M.; Basta, G.; Calafiore, R.; Rossi, C. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: In vitro effects on isolated neonatal porcine pancreatic cell clusters. J. Control. Release 2005, 107, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, S.; Blasi, P.; Ricci, M.; Rossi, C. Biodegradable microspheres as carriers for native superoxide dismutase and catalase delivery. AAPS PharmSciTech 2004, 5, e51. [Google Scholar] [CrossRef] [Green Version]
- Kartha, S.; Yan, L.; Weisshaar, C.L.; Ita, M.E.; Shuvaev, V.V.; Muzykantov, V.R.; Tsourkas, A.; Winkelstein, B.A.; Cheng, Z. Superoxide Dismutase-Loaded Porous Polymersomes as Highly Efficient Antioxidants for Treating Neuropathic Pain. Adv. Healthcare Mater. 2017, 6, 1700500. [Google Scholar] [CrossRef]
- Beckman, J.S.; Minor, R.L.; White, C.W.; Repine, J.E.; Rosen, G.M.; Freeman, B.A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J. Biol. Chem. 1988, 263, 6884–6892. [Google Scholar]
- Jiang, Y.; Brynskikh, A.M.; Manickam, D.S.; Kabanov, A.V. SOD1 nanozyme salvages ischemic brain by locally protecting cerebral vasculature. J. Control. Release 2015, 213, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Arounleut, P.; Rheiner, S.; Bae, Y.; Kabanov, A.V.; Milligan, C.; Manickam, D.S. SOD1 nanozyme with reduced toxicity and MPS accumulation. J. Control. Release 2016, 231, 38–49. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, C.-F.; Tsai, W.-B. Fabrication of Chlorophyll-Incorporated Nanogels for Potential Applications in Photothermal Cancer Therapy. ACS Omega 2018, 3, 16057–16062. [Google Scholar] [CrossRef]
- Chang, R.; Tsai, W.-B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef] [Green Version]
- Wonoputri, V.; Gunawan, C.; Liu, S.; Barraud, N.; Yee, L.H.; Lim, M.; Amal, R. Copper Complex in Poly(vinyl chloride) as a Nitric Oxide-Generating Catalyst for the Control of Nitrifying Bacterial Biofilms. ACS Appl. Mater. Interfaces 2015, 7, 22148–22156. [Google Scholar] [CrossRef] [PubMed]
- Wonoputri, V.; Gunawan, C.; Liu, S.; Barraud, N.; Yee, L.H.; Lim, M.; Amal, R. Iron Complex Facilitated Copper Redox Cycling for Nitric Oxide Generation as Nontoxic Nitrifying Biofilm Inhibitor. ACS Appl. Mater. Interfaces 2016, 8, 30502–30510. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal–Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Li, W.; Liu, J.; Chen, J.; Fan, Y.; Weng, Y. Substrate Independent Liquid Phase Epitaxy of HKUST-1 as Anticoagulant and Antimicrobial Coating. Adv. Mater. Interfaces 2020, 7, 1902011. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, S.; Meng, X.; Sun, B.; Zhang, X.; Zhang, L.; Liu, Y.; Lin, M.; Zhang, H.; et al. Multidrug resistant tumors-aimed theranostics on the basis of strong electrostatic attraction between resistant cells and nanomaterials. Biomater. Sci. 2019, 7, 4990–5001. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Pan, Q.; Zhang, B.; Wan, S.; Li, S.; Luo, K.; Pu, Y.; He, B. Highly Stable, Coordinated Polymeric Nanoparticles Loading Copper(II) Diethyldithiocarbamate for Combinational Chemo/Chemodynamic Therapy of Cancer. Biomacromolecules 2019, 20, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Sun, R.; Geng, Y.; Zhou, Q.; Piao, Y.; Xie, T.; Zhou, R.; Shen, Y. N-Oxide Polymer-Cupric Ion Nanogels Potentiate Disulfiram for Cancer Therapy. Biomater. Sci. 2020, 8, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Boodram, J.N.; Cressey, P.B.; Lu, C.; Bruno, P.M.; Hemann, M.T.; Suntharalingam, K. The Breast Cancer Stem Cell Potency of Copper(II) Complexes Bearing Nonsteroidal Anti-Inflammatory Drugs and Their Encapsulation Using Polymeric Nanoparticles. Dalton Trans. 2016, 45, 17867–17873. [Google Scholar] [CrossRef] [Green Version]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Kumar, R.S.; Arunachalam, S. DNA binding and antimicrobial studies of some polyethyleneimine-copper(II) complex samples containing 1,10-phenanthroline and L-threonine as co-ligands. Polyhedron 2007, 26, 3255–3262. [Google Scholar] [CrossRef]
- Kumar, R.S.; Arunachalam, S. DNA binding and antimicrobial studies of polymer–copper(II) complexes containing 1,10-phenanthroline and L-phenylalanine ligands. Eur. J. Med. Chem. 2009, 44, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Arunachalam, S.; Periasamy, V.S.; Preethy, C.P.; Riyasdeen, A. DNA binding and biological studies of some novel water-soluble polymer-copper(II)-phenanthroline complexes. Eur. J. Med. Chem. 2008, 43, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Sasikala, K.; Arunachalam, S. DNA interaction of some polymer–copper(II) complexes containing 2,2′-bipyridyl ligand and their antimicrobial activities. J. Inorg. Biochem. 2008, 102, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Periasamy, V.S.; Paul, C.P.; Riyasdeen, A.; Arunachalam, S.; Akbarsha, M.A. Cytotoxic effect of a polymer–copper(II) complex containing 2,2′-bipyridyl ligand on human lung cancer cells. Med. Chem. Res. 2011, 20, 726–731. [Google Scholar] [CrossRef]
- Hausman, R.; Gullinkala, T.; Escobar, I.C. Development of Low-Biofouling Polypropylene Feedspacers for Reverse Osmosis. J. Appl. Polym. Sci. 2009, 114, 3068–3073. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Sweileh, B.; Taha, S.O.; Mahmoud, R.; Taha, M.O. Preparation of Polyester-Based Metal-Cross Linked Polymeric Composites as Novel Materials Resistant to Bacterial Adhesion and Biofilm Formation. Molecules 2011, 16, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, M.I.A.; Ahamad, I.; Iqbal, S.; Malik, M.A.; Dar, O.A.; Akram, M.d.K.; Fatma, T.; Hashmi, A.A. Fabrication of metal incorporated polymer composite: An excellent antibacterial agent. J. Mol. Struct. 2021, 1225, 129091. [Google Scholar] [CrossRef]
- Galliani, M.; Signore, G. Poly(Lactide-Co-Glycolide) Nanoparticles Co-Loaded with Chlorophyllin and Quantum Dots as Photodynamic Therapy Agents. ChemPlusChem 2019, 84, 1653–1658. [Google Scholar] [CrossRef]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual antibacterial activities of a chitosan-modified upconversion photodynamic therapy system against drug-resistant bacteria in deep tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef]
- Arimoto-Kobayashi, S.; Harada, N.; Tokunaga, R.; Odo, J.-I.; Hayatsu, H. Adsorption of mutagens to chlorophyllin–chitosan, an insoluble form of chlorophyllin. Mutat. Res. 1997, 381, 243–249. [Google Scholar] [CrossRef]
- Celik, O.; Akbuğa, J. Preparation of superoxide dismutase loaded chitosan microspheres: Characterization and release studies. Eur. J. Pharm. Biopharm. 2007, 66, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-M.; He, N.-P.; He, Y.-F.; Xie, Y.-T.; Wang, Y.-P.; Tsuchida, E. The preparation of nano-scope chitosanoligomer copper complexes and their interaction with DNA. Polym. Adv. Technol. 2005, 16, 638–641. [Google Scholar] [CrossRef]
- Wang, R.-M.; He, N.-P.; Song, P.-F.; He, Y.-F.; Ding, L.; Lei, Z.-Q. Preparation of nano-chitosan Schiff-base copper complexes and their anticancer activity. Polym. Adv. Technol. 2009, 20, 959–964. [Google Scholar] [CrossRef]
- Malekshaha, R.E.; Shakerib, F.; Khaleghianb, A.; Salehi, M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as Pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Calderón, J.E.; Macías-Rosales, L.; Gracia-Mora, I.; Ruiz-Azuara, L.; Faustino-Vega, A.; Gracia-Mora, J.; Bernad-Bernad, M.J. Effect of casiopein III-ia loaded into chitosan nanoparticles on tumor growth inhibition. J. Drug Deliv. Sci. Technol. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Gritsch, L.; Liverani, L.; Lovell, C.; Boccaccini, A.R. Polycaprolactone Electrospun Fiber Mats Prepared Using Benign Solvents: Blending with Copper(II)-Chitosan Increases the Secretion of Vascular Endothelial Growth Factor in a Bone Marrow Stromal Cell Line. Macromol. Biosci. 2020, 20, 1900355. [Google Scholar] [CrossRef]
- Ren, X.; Yang, C.; Zhang, L.; Li, S.; Shi, S.; Wang, R.; Zhang, X.; Yue, T.; Sun, J.; Wang, J. Copper metal–organic frameworks loaded on chitosan film for the efficient inhibition of bacteria and local infection therapy. Nanoscale 2019, 11, 11830–11838. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Ma, Y.; Yua, H.; Liua, W.; Qin, Y.; Xinga, R.; Li, P. Integrated proteomics and metabolomics analysis reveals the antifungal mechanism of the C-coordinated O-carboxymethyl chitosan Cu (II) complex. Int. J. Biol. Macromol. 2020, 155, 1491–1509. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Lamponi, S.; Mitola, S.; Ziche, M.; Morbidelli, L.; Bussolino, F. Cu(II) and Zn(II) complexes with hyaluronic acid and its sulphated derivative: Effect on the motility of vascular endothelial cells. J. Inorg. Biochem. 2000, 81, 229–237. [Google Scholar] [CrossRef]
- Barbucci, R.; Leone, G.; Magnani, A.; Montanaro, L.; Arciola, C.R.; Peluso, G.; Petillo, O. Cu2+- and Ag+-complexes with a hyaluronane-based hydrogel. J. Mater. Chem. 2002, 12, 3084–3092. [Google Scholar] [CrossRef]
- Hu, C.; Cai, L.; Liu, S.; Liu, Y.; Zhou, Y.; Pang, M. Copper-doped Nanoscale Covalent Organic Polymer for Augmented Photo-/Chemodynamic Synergistic Therapy and Immunotherapy. Bioconjug. Chem. 2020, 31, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Matsui, Y.; Ikada, Y. Growth factor release from amylopectin hydrogel based on copper coordination. J. Control. Release 1998, 56, 135–148. [Google Scholar] [CrossRef]
- Tabata, Y.; Noda, Y.; Matsui, Y.; Ikada, Y. Targeting of tumor necrosis factor to tumor by use of dextran and metal coordination. J. Control. Release 1999, 59, 187–196. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug. Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Hefni, H.H. Synthesis and antimicrobial activity of polysaccharide alginate derived cationic surfactant–metal(II) complexes. Int. J. Biol. Macromol. 2016, 82, 562–572. [Google Scholar] [CrossRef]
- Suzuki, R.; Inoue, Y.; Murata, I.; Nomura, H.; Isshiki, Y.; Hashimoto, M.; Kudo, Y.; Kitagishi, H.; Kondo, S.; Kanamoto, I. Preparation, characterization, and study of the antimicrobial activity of a Hinokitiol-copper(II)/gamma-cyclodextrin ternary complex. J. Mol. Struct. 2019, 1194, e27. [Google Scholar] [CrossRef]
- Xia, N.; Wan, W.; Zhu, S.; Liu, Q. Preparation of crystalline nanocellulose/hydroxypropyl β cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef]
- Gómez-Machuca, H.; Quiroga-Campano, C.; Zapata-Torres, G.; Jullian, C. Influence of DMβCD on the Interaction of Copper(II) Complex of 6-Hydroxychromone-3-carbaldehyde-3-hydroxybenzoylhydrazine with ctDNA, ACS Omega 2020, 5, 6928–6936. [CrossRef] [Green Version]
- Demirdogen, R.E.; Kilic, D.; Emen, F.M.; Așkar, Ș.; Karaçolak, A.I.; Yesilkaynak, T.; Ihsan, A. Novel antibacterial cellulose acetate fibers modified with 2-fluoropyridine complexes. J. Mol. Struct. 2020, 1204, 127537. [Google Scholar] [CrossRef]
- Xua, Y.; Shia, Y.; Leia, F.; Dai, L. A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr. Polym. 2020, 230, 115671. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tomala, W.; Żurańska, B.; Dobrowolska, A.; Piasecki, A.; Czaczyk, K.; Ehrlich, H.; Jesionowski, T. Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity. Int. J. Mol. Sci. 2016, 17, 1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassman, P.M.; Walsh, L.R.; Villa, C.H.; Marcos-Contreras, O.A.; Hood, E.D.; Muzykantov, V.R.; Greineder, C.F. Molecularly Engineered Nanobodies for Tunable Pharmacokinetics and Drug Delivery. Bioconjug. Chem. 2020, 31, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Khoshnejad, M.; Pulsipher, K.W.; Kiseleva, R.Y.; Arguiri, E.; Cheung-Lau, J.C.; LeFort, K.M.; Christofidou-Solomidou, M.; Stan, R.V.; Dmochowski, I.J.; et al. Spatially controlled assembly of affinity ligand and enzyme cargo enables targeting ferritin nanocarriers to caveolae. Biomaterials 2018, 185, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Kiseleva, R.Y.; Arguiri, E.; Villa, C.H.; Muro, S.; Christofidou-Solomidou, M.; Stan, R.V.; Muzykantov, V.R. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J. Control. Release 2018, 272, 1–8. [Google Scholar] [CrossRef]
- Shuvaev, V.V.; Muro, S.; Arguiri, E.; Khoshnejad, M.; Tliba, S.; Christofidou-Solomidou, M.; Muzykantov, V.R. Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J. Control. Release 2016, 234, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Xu, H.; Xiao, B.; Li, D.; Zhou, Z.; Liu, X.; Tang, J.; Shen, Y. Albumin stabilized metal-organic nanoparticles for effective delivery of metal complex anticancer drugs. ACS Appl. Mater. Interfaces 2018, 10, 34974–34982. [Google Scholar] [CrossRef]
- Llamazares, C.; Sanz del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; Javier de la Mata, F.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Bosch, P.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Kukeva, R.; Stoyanova, R.; Grabchev, I. New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials 2019, 12, 3020. [Google Scholar] [CrossRef] [Green Version]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Synthesis, spectral characterization and in vitro antimicrobial activity in liquid medium and applied on cotton fabric of a new PAMAM metallodendrimer. Int. J. Polym. Anal. Char. 2018, 23, 45–57. [Google Scholar] [CrossRef]
- Grabcheva, I.; Vasileva-Tonkovab, E.; Stanevac, D.; Boschd, P.; Kukevae, R.; Stoyanova, R. Impact of the Cu(II) and Zn(II) ions on the functional properties of new PAMAM metallodendrimers. New J. Chem. 2018, 42, 7853–7862. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Phosphorus dendrimers functionalised with nitrogen ligands, for catalysis and biology. Dalton Trans. 2019, 48, 7483–7493. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.M.; Aubert, G.; Laurent, R.; Caminade, A.-M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.-P. Original Multivalent Copper(II)-Conjugated Phosphorus Dendrimers and Corresponding Mononuclear Copper(II) Complexes with Antitumoral Activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.F.; El Brahmi, N.; Cangiotti, M.; Coppola, C.; Buccella, F.; Cresteil, T.; Mignani, S.; Caminade, A.M.; Costes, J.P.; Majoral, J.P. Comparative EPR studies of Cu(II)-conjugated phosphorous-dendrimers in the absence and presence of normal and cancer cells. RSC Adv. 2014, 4, 36573–36583. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, L.; Yin, F.; Zhu, Y.; Shen, M.; Wang, H.; Du, L.; Mignani, S.; Majorale, J.-P.; Shi, X. Phosphorus dendrimer-based copper(II) complexes enable ultrasound-enhanced tumor theranostics. Nano Today 2020, 33, 100899. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original Multivalent Gold(III) and Dual Gold(III)−Copper(II) Conjugated Phosphorus Dendrimers as Potent Antitumoral and Antimicrobial Agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef]
- Canonico, B.; Carloni, R.; Sanz Del Olmo, N.; Papa, S.; Nasoni, M.G.; Fattori, A.; Cangiotti, M.; De La Mata, F.J.; Ottaviani, M.F.; García-Gallego, S. Fine-Tuning the Interaction and Therapeutic Effect of Cu(II) Carbosilane Metallodendrimers in Cancer Cells: An in Vitro Electron Paramagnetic Resonance Study. Mol. Pharm. 2020, 17, 2691–2702. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; Javier de la Mata, F. Insight into the antitumor activity of carbosilane Cu(II)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Holota, M.; Michlewska, S.; Gómez, R.; Ortega, P.; Ionov, M.; Javier de la Mata, F.; Bryszewska, M. Copper (II) Metallodendrimers Combined with Pro-Apoptotic siRNAs as a Promising Strategy Against Breast Cancer Cells. Pharmaceutics 2020, 12, 727. [Google Scholar] [CrossRef]
- Holota, M.; Magiera, J.; Michlewska, S.; Kubczak, M.; Sanz del Olmo, N.; García-Gallego, S.; Ortega, P.; Javier de la Mata, F.; Ionov, M.; Bryszewska, M. In Vitro Anticancer Properties of Copper Metallodendrimers. Biomolecules 2019, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Zhang, Y.; Zhaoa, Q.; Xie, Y.; Luo, R.; Yang, P.; Weng, Y. Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents. Biomaterials 2019, 204, 36–45. [Google Scholar] [CrossRef]
- Oliveira, G.R.; do Amaral, L.J.; Giovanela, M.; da Silva Crespo, J.; Fetter, G.; Rivera, J.A.; Sampieri, A.; Bosch, P. Bactericidal performance of chlorophyllin-copper hydrotalcite compounds. Water Air Soil. Pollut. 2015, 226, 1–12. [Google Scholar] [CrossRef]
- Azimi, S.; Behin, J.; Abiri, R.; Rajabi, L.; Derakhshan, A.A.; Karimnezhad, S.H. Synthesis, characterization and antibacterial activity of chlorophyllin functionalized graphene oxide nanostructures. Sci. Adv. Mater. 2014, 6, 771–781. [Google Scholar] [CrossRef]
- Singh, S.; Dubey, V.K. Multiwalled Carbon Nanotube-Superoxide Dismutase Conjugate Towards Alleviating Induced Oxidative Stress. Int. J. Pept. Res. Ther. 2016, 22, 171–177. [Google Scholar] [CrossRef]
- Binevski, P.V.; Balabushevich, N.G.; Uvarova, V.I.; Vikulina, A.S.; Volodkin, D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids Surf. B Biointerfaces 2019, 181, 437–449. [Google Scholar] [CrossRef] [PubMed]
- López, T.; Ortiz, E.; Guevara, P.; Gómez, E.; Novaro, O. Physicochemical characterization of functionalized nanostructured-titania as a carrier of copper complexes for cancer treatment. Mater. Chem. Phys. 2014, 146, 37–49. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, 1900669. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Busa, P.; Lin, S.-X.; Deng, J.-P.; Mou, C.-Y.; Lee, C.-H. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J. Photochem. Photobiol. B 2017, 169, 124–133. [Google Scholar] [CrossRef]
- Tahmasbi, L.; Sedaghat, T.; Motamedi, H.; Kooti, M. Mesoporous silica nanoparticles supported copper(II) and nickel(II) Schiff base complexes: Synthesis, characterization, antibacterial activity and enzyme immobilization. J. Solid State Chem. 2018, 258, 517–525. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-García, D.; Ardiles, P.R.; Díaz-Sánchez, M.; Mena-Palomo, I.; del Hierro, I.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Copper-functionalized nanostructured silica-based systems: Study of the antimicrobial applications and ROS generation against gram positive and gram negative bacteria. J. Inorg. Biochem. 2020, 203, 110912. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, M.; Daier, V.; Camí, G.; Rivière, E.; Hureau, C.; Signorella, S. Preparation, characterization and activity of CuZn and Cu2 superoxide dismutase mimics encapsulated in mesoporous silica. J. Inorg. Biochem. 2020, 207, 111050. [Google Scholar] [CrossRef] [PubMed]
- Sheena, T.S.; Dhivya, R.; Rajiu, V.; Jeganathan, K.; Palaniandavar, M.; Mathan, G.; Akbarsha, M.A. Folate-engineered mesoporous silica-encapsulated copper (II) complex [Cu (L)(dppz)]+: An active targeting cell-specific platform for breast cancer therapy. Inorg. Chim. Acta 2020, 510, 119783. [Google Scholar] [CrossRef]
- Sudareva, N.; Suvorova, O.; Saprykina, N.; Vilesov, A.; Bel’tyukov, P.; Petunov, S. Alginate-containing systems for oral delivery of superoxide dismutase. Comparison of various configurations and their properties. J. Microencapsul. 2016, 33, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.K.; Palanimuthu, D.; Somasundaram, K.; Samuelson, A.G. Biotin Decorated Gold Nanoparticles for Targeted Delivery of a Smart-Linked Anticancer Active Copper Complex: In Vitro and In Vivo Studies. Bioconjug. Chem. 2016, 27, 2874–2885. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Ilyas, K.; Dlouhý, I.; Siska, F.; Boccaccini, A.R. Electrophoretic Deposition of Copper(II)–Chitosan Complexes for Antibacterial Coatings. Int. J. Mol. Sci. 2020, 21, 2637. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.; Sedaghat, T.; Motamedi, H.; Azadi, R. Anchoring of Cu (II)-Schiff base complex on magnetic mesoporous silica nanoparticles: Catalytic efficacy in one-pot synthesis of 5-substituted-1H-tetrazoles, antibacterial activity evaluation and immobilization of α-amylase. Appl. Organometal. Chem. 2020, e5572. [Google Scholar] [CrossRef]


| Complex/Conjugated Moiety | Matrix | Activity | Other Tests | Ref |
|---|---|---|---|---|
| Liposomal Formulations | ||||
 (9) | ePC ePC:DMPC:DPPC: DSPE-PEG DMPC:Chol:DSPE-PEG DMPC:CHEMS: DSPE-PEG2000 | A431 (IC50: 10/8.3 μM), HaCat (IC50: 5.3/5.8 μM), MNT-1 ((IC50: 4.8/4.4 μM)), B16F10 (IC50: 4.5/5.1 μM), C26 (IC50: 5.8/4.4 μM) male BALB/c mice B16F10 (IC50: 5.1/2.1/2.7 μM) | In vitro hemolysis, hepatotoxicity Hepatic biochemical parameters, caspase activity | [45,46] |
 X is Cl (10), NCS (11), NCO (12) | CHLN:PC | M. tuberculosis (MIC: 0.397, 0.219, 0.313 μg/mL) S. aureus ATCC 25,923 (MIC: 250, 500, 125 μg/mL), E. coli ATCC 25,922 (MIC: 125, 125, 500 μg/mL) | Vero cell line cytotoxicity ((IC50 109.5–319.3 μg/mL), macrophage, Artemia salina (brine shrimp) | [47,48] |
 (13) | DSPC:CHOL: DSPE-PEG2000 | Female CD-1 mice | [49] | |
 (14) | DPPC:HSPC | HT-29 (IC50: 0.2–10.1 μM) C26 (IC50: 0.2–4.2 μM) C26 grafted to BALB/c mice | [51] | |
 (15) | DSPC:CHOL (55:45 molar ratio) | parenteral administration | [52] | |
| Polymer formulations | ||||
 (16) | PVC | Biofilm produced by nitrifying bacteria inhibition | [72,73] | |
 Basic moiety in (17) | PPCN PVC | Wound healing in diabetic mice Biofilm of S. aureus, E. coli inhibition | Cell migration, angiogenesis and collagen deposition | [74,75] |
 (18) | PEGPLGA | HMLER (IC50: 7.38 μM) HMLERshEcad (IC50: 2.21 μM) | ROS production COX-2 inhibition | [79] |
 (19) | BPEI | S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans (diameter of inhibition zone) | CT DNA interaction | [81] |
 (20) | BPEI | S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans (diameter of inhibition zone) | CT DNA interaction | [82] |
 (21) | BPEI | S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans (diameter of inhibition zone) NCI-H460 cells (IC50: 13.2 μg/mL) | CT DNA interaction | [83] |
 (22) | BPEI | S. aureus, B. subtilis, E. coli, P. aeruginosa, C. albicans (diameter of inhibition zone) NCI-H460 (IC50: 90–95 μg/mL) | CT DNA interaction | [84,85] |
 (23) X: CH3COO, (24) ClO4 | Chitosan Schiff base with 2-hydroxy-3-metoxybenzaldehyde | K562 (IC50: 1 μg/mL) MG-63 (IC50: 25 μg/mL) | Apoptosis studies | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830. https://doi.org/10.3390/molecules25245830
Badea M, Uivarosi V, Olar R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules. 2020; 25(24):5830. https://doi.org/10.3390/molecules25245830
Chicago/Turabian StyleBadea, Mihaela, Valentina Uivarosi, and Rodica Olar. 2020. "Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix" Molecules 25, no. 24: 5830. https://doi.org/10.3390/molecules25245830