Structure and Rheological Properties of Glycerol Monolaurate-Induced Organogels: Influence of Hydrocolloids with Different Surface Charge
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of OG in the Absence and Presence of Hydrocolloids
2.2. Rheological Behavior of OGs
2.2.1. Steady Shear Rheological Behavior of OGs and MOGs
2.2.2. Frequency-Dependent Rheological Behavior of MOGs
2.2.3. Temperature-Dependent Rheological Behavior of MOGs
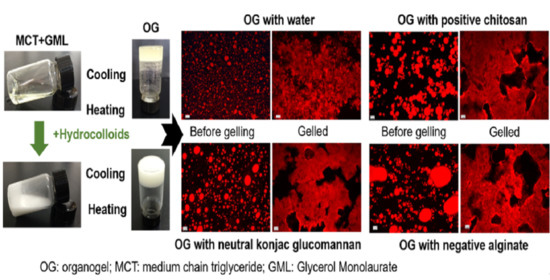
2.3. Microstructure of OGs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of OGs
3.2.2. Preparation of Modified OGs (MOGs)
3.3. Oil holding Capacity and Gelling Time
3.4. Texture Characterization
3.5. Rheological Properties of OGs
3.6. Microstructure Characterization
3.6.1. Fluorescent Microscopy
3.6.2. Scanning Electronic Microscopy
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moschakis, T.; Panagiotopoulou, E.; Katsanidis, E. Sunflower oil organogels and organogel-in-water emulsions (part I): Microstructure and mechanical properties. LWT 2016, 73, 153–161. [Google Scholar] [CrossRef]
- Aliasl Khiabani, A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Chaves, K.F.; Barrera-Arellano, D.; Ribeiro, A.P.B. Potential application of lipid organogels for food industry. Food Res. Int. 2018, 105, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Developing organogel-based Pickering emulsions with improved freeze-thaw stability and hesperidin bioaccessibility. Food Hydrocoll. 2019, 93, 68–77. [Google Scholar] [CrossRef]
- Mao, L.; Lu, Y.; Cui, M.; Miao, S.; Gao, Y. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.F.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and mechanical properties of organogels: Role of oil and gelator molecular structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, P.; Tabibiazar, M.; Roufegarinejad, L.; Babazadeh, A. Development of behenic acid-ethyl cellulose oleogel stabilized Pickering emulsions as low calorie fat replacer. Int. J. Biol. Macromol. 2020, 150, 974–981. [Google Scholar] [CrossRef]
- Li, L.; Wan, W.; Cheng, W.; Liu, G.; Han, L. Oxidatively stable curcumin-loaded oleogels structured by β-sitosterol and lecithin: Physical characteristics and release behaviour in vitro. Int. J. Food Sci. Technol. 2019, 54, 2502–2510. [Google Scholar] [CrossRef]
- Puscas, A.; Muresan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, M.; Goli, S.A.H.; Fayaz, G. Exploitation of high-intensity ultrasound to modify the structure of olive oil organogel containing propolis wax. Int. J. Food Sci. Technol. 2019, 54, 509–515. [Google Scholar] [CrossRef]
- Gokce, E.H.; Yurdasiper, A.; Korkmaz, E.; Ozer, O. A novel preparation method for organogels: High-speed homogenization and micro-irradiation. AAPS PharmSciTech 2013, 14, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Han, X.; Zhu, G.; Ding, Y.; Miao, Y.; Wang, K.; Zhang, H.; Wang, Y.; Liu, S.-B. Selective catalytic synthesis of glycerol monolaurate over silica gel-based sulfonic acid functionalized ionic liquid catalysts. Chem. Eng. J. 2019, 359, 733–745. [Google Scholar] [CrossRef]
- Lopez-Martínez, A.; Charó-Alonso, M.A.; Marangoni, A.G.; Toro-Vazquez, J.F. Monoglyceride organogels developed in vegetable oil with and without ethylcellulose. Food Res. Int. 2015, 72, 37–46. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. Properties and Stability of Hazelnut Oil Organogels with Beeswax and Monoglyceride. J. Am. Oil Chem. Soc. 2014, 91, 1007–1017. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Banerjee, I.; Pramanik, K.; Basak, P.; Pal, K. Core–shell-type organogel–alginate hybrid microparticles: A controlled delivery vehicle. Chem. Eng. J. 2015, 264, 134–145. [Google Scholar] [CrossRef]
- Sołowiej, B.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Texture, rheology and meltability of processed cheese analogues prepared using rennet or acid casein with or without added whey proteins. Int. Dairy J. 2014, 37, 87–94. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Barbosa de Souza Ferreira, S.; Bruschi, M.L.; Britten, M.; Matumoto-Pintro, P.T. Effect of commercial konjac glucomannan and konjac flours on textural, rheological and microstructural properties of low fat processed cheese. Food Hydrocoll. 2016, 60, 308–316. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.K.; Pal, K. Stearate organogel-gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, L.; Que, F.; Feng, F.; Zhang, H. Physical characterization and antimicrobial evaluation of glycerol monolaurate organogels. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 19–25. [Google Scholar] [CrossRef]
- Do Nascimento, G.E.; Simas-Tosin, F.F.; Iacomini, M.; Gorin, P.A.; Cordeiro, L.M. Rheological behavior of high methoxyl pectin from the pulp of tamarillo fruit (Solanum betaceum). Carbohydr. Polym. 2016, 139, 125–130. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.A.; Mansour, M.; El-Assal, M.I.A.; Ishak, R.A.H.; Mortada, N.D. Delineating penetration enhancer-enriched liquid crystalline nanostructures as novel platforms for improved ophthalmic delivery. Int. J. Pharm. 2020, 582, 119313. [Google Scholar] [CrossRef]
- Rocha-Amador, O.G.; Gallegos-Infante, J.A.; Huang, Q.; González-Laredo, R.F. Effect of Glycosylation Degree of Quercetin on Its In Vitro Bioaccessibility in Food Grade Organogels. Int. J. Food Eng. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Han, L.-J.; Li, L.; Zhao, L.; Li, B.; Liu, G.-Q.; Liu, X.-Q.; Wang, X.-D. Rheological properties of organogels developed by sitosterol and lecithin. Food Res. Int. 2013, 53, 42–48. [Google Scholar] [CrossRef]
- Nishinari, K. Some Thoughts on the Definition of a Gel. In Gels: Structures, Properties, and Functions; Tokita, M., Nishinari, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 87–94. [Google Scholar]
- Li, J.; Wang, Y.; Jin, W.; Zhou, B.; Li, B. Application of micronized konjac gel for fat analogue in mayonnaise. Food Hydrocoll. 2014, 35, 375–382. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, Y.; Wang, Y.; Liu, Y. Oleogels from sodium stearoyl lactylate-based lamellar crystals: Structural characterization and bread application. Food Chem. 2019, 292, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Influencing the crystallization behavior of binary mixtures of stearyl alcohol and stearic acid (SOSA) using ethylcellulose. Food Res. Int. 2017, 91, 1–10. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, M.; Won, D.-A.; Tae, G. Preparation of nanosize alginate gel using pluronic-based nano-carrier as a template. Eur. Polym. J. 2015, 72, 632–641. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Samples | Gelation Time | Stability (%OBC) | Separation | Texture Characteristics | ||
|---|---|---|---|---|---|---|
| Firmness (g) | Stickiness (g) | Adhesiveness (g·sec) | ||||
| OG | 21 min | 65.3% | Obvious separation | 213.04 ± 22.88 a | −165.03 ± 25.53 c | −129.80 ± 20.91 a |
| CSOG | 1 min 42 s | 93.9% | No separation | 470.25 ± 40.96 d | −333.55 ± 84.47 a | −93.74 ± 11.35 a |
| KGMOG | 1 min 48 s | 99.2% | No separation | 377.02 ± 49.37 c | −264.31 ± 57.19 ab | −121.59 ± 51.54 a |
| SAOG | 1 min 40 s | 84.1% | Slight separation | 297.79 ± 40.17 b | −227.33 ± 41.78 bc | −105.54 ± 25.93 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wu, S.; Liu, S.; Li, B.; Li, Y. Structure and Rheological Properties of Glycerol Monolaurate-Induced Organogels: Influence of Hydrocolloids with Different Surface Charge. Molecules 2020, 25, 5117. https://doi.org/10.3390/molecules25215117
Zhao R, Wu S, Liu S, Li B, Li Y. Structure and Rheological Properties of Glycerol Monolaurate-Induced Organogels: Influence of Hydrocolloids with Different Surface Charge. Molecules. 2020; 25(21):5117. https://doi.org/10.3390/molecules25215117
Chicago/Turabian StyleZhao, Runan, Shan Wu, Shilin Liu, Bin Li, and Yan Li. 2020. "Structure and Rheological Properties of Glycerol Monolaurate-Induced Organogels: Influence of Hydrocolloids with Different Surface Charge" Molecules 25, no. 21: 5117. https://doi.org/10.3390/molecules25215117