Ultrasound–Vortex-Assisted Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Diode Array Detection for Determining UV Filters in Cosmetics and the Human Stratum Corneum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
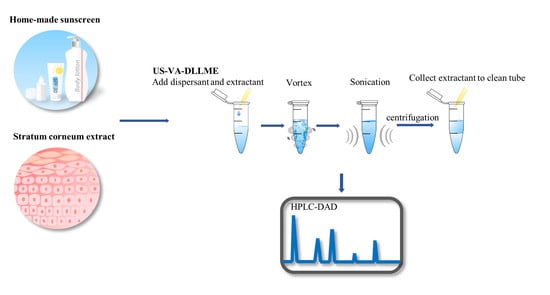
2.2. The US–VA–DLLME Procedure
2.3. Instrumentation and Conditions
2.4. Method Validation
2.5. Cosmetics Application
2.6. Evaluating the Sunscreen-Removal Efficiency
2.7. Evaluating the UV Filter Concentration in Human Skin after Spreading a Sunscreen Sample on the Forearm
2.7.1. Single Applications of Sunscreen to the Skin with Different Exposure Times (0.5, 1, 4, and 8 h)
2.7.2. Double Application of the Sunscreen to the Skin (4 + 4 h)
3. Results and Discussion
3.1. Optimizing the US–VA–DLLME Procedure
3.1.1. Effect of the Dispersant
3.1.2. Effect of the Extractant
3.1.3. Effect of Vortexing and Ultrasonication
3.2. Method Validation
3.3. Cosmetics Analysis
3.4. Evaluating the Concentrations of UV Filter Residues in Human Skin after Exposure to Sunscreen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Union 2020. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.results&annex_v2=VI&search (accessed on 8 April 2020).
- Taiwan Food and Drug Administration 2019. Available online: https://www.fda.gov.tw/TC/newsContent.aspx?cid=3&id=25268 (accessed on 8 April 2020).
- U.S. Food and Drug Administration 2020. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=53ff6cdf9fce5c7cdab0b2972b9c8c59&mc=true&node=pt21.5.352&rgn=div5#se21.5.352_152 (accessed on 8 April 2020).
- Ocaña-Rios, I.; Peña-Alvarez, A.; Zuñiga-Perez, I.; Loeza-Fuentes, E. Trace analysis of UV filters and musks in living fish by in vivo SPME-GC-MS. Anal. Bioanal. Chem. 2019, 411, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Facorro, R.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.M.; Llompart, M. Determination of fifteen water and fat-soluble UV filters in cosmetics by pressurized liquid extraction followed by liquid chromatography tandem mass spectrometry. Anal. Methods 2016, 8, 6787–6794. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Gallardo-Torres, M.E.; Ballesteros, O.; Díaz, C.; Pérez, J.; Navalón, A.; Fernández, M.F.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Timm, A.; Blaneya, L. Simultaneous determination of UV-filters and estrogens in aquatic invertebrates by modified quick, easy, cheap, effective, rugged, and safe extraction and liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2017, 1509, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Maia Campos, P.M.B.G. Development of a HPLC method for determination of four UV filters in sunscreen and its application to skin penetration studies. Biomed. Chromatogr. 2017, 31, e4029. [Google Scholar] [CrossRef]
- Vosough, M.; Shekari, N.; Salemi, A.; Heidar, K.T. Chemometrics-assisted fast-elution HPLC–DAD for the quantification of selected UV filters and parabens in suncare formulations. J. AOAC Int. 2017, 100, 377–386. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of dispersive liquid–liquid microextraction method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Yiantzi, E.; Psillakis, E.; Tyrovola, K.; Kalogerakis, N. Vortex-assisted liquid–liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta 2010, 80, 2057–2062. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trend Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Vila, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Ultrasound-assisted emulsification microextraction followed by gas chromatography–mass spectrometry and gas chromatography–tandem mass spectrometry for the analysis of UV filters in water. Microchem. J. 2016, 124, 530–539. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, J.; Liu, Y.; Zhao, X.; Cao, Z.; Xia, L.; Li, G.; Sun, Z.; Zhang, S.; et al. A rapid, accurate and sensitive method with the new stable isotopic tags based on microwave-assisted dispersive liquid-liquid microextraction and its application to the determination of hydroxyl UV filters in environmental water samples. Talanta 2017, 167, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid–liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography–ion trap mass spectrometry. Anal. Chim. Acta 2013, 769, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trend Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. TrAC Trend Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction. TrAC Trend Anal. Chem. 2019, 120, 115651. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trend Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, D.; Gates, B.C.; Rahimpour, M.R. Upgrading of liginin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar]
- Scalia, S.; Battaglioli, S.; Bianchi, A. In vivo human skin penetration of the UV filter ethylhexyl triazone: Effect of lipid microparticle encapsulation. Skin Pharmacol. Physiol. 2019, 32, 22–31. [Google Scholar]
- Klimová, Z.; Hojerová, J.; Beránková, M. Skin absorption and human exposure estimation of three widely discussed UV filters in sunscreens—In vitro study mimicking real-life consumer habits. Food Chem. Toxicol. 2015, 83, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Hojerová, J.; Peráčková, Z.; Beránková, M. Margin of safety for two UV filters estimated by in vitro permeation studies mimicking consumer habits: Effects of skin shaving and sunscreen reapplication. Food Chem. Toxicol. 2017, 103, 66–78. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. 2007, 27, 1–9. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s solvent selection guide: A step toward more sustainable processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C.; et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA 2019, 321, 2082–2091. [Google Scholar] [CrossRef] [Green Version]
- Hiller, J.; Klotz, K.; Meyer, S.; Uter, W.; Hof, K.; Greiner, A.; Göen, T.; Drexler, H. Systemic availability of lipophilic organic UV filters through dermal sunscreen exposure. Environ. Int. 2019, 132, 105068. [Google Scholar] [CrossRef]
- Klotz, K.; Hof, K.; Hiller, J.; Göen, T.; Drexler, H. Quantification of prominent organic UV filters and their metabolites in human urine and plasma samples. J. Chromatogr. B 2019, 1125, 121706. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Chromolith | ZORBAX 300SB | XBridge BEH | CORTECS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemistry | C18 | C18 | C18 | C18 | ||||||||||||
| I.D. (mm) | 2 | 2.1 | 2.1 | 2.1 | ||||||||||||
| L (mm) | 100 | 100 | 50 | 50 | ||||||||||||
| Particle Size (µm) | - | 3.5 | 2.5 | 2.7 | ||||||||||||
| UV filters | k | α | N/m | Rs | k | α | N/m | Rs | k | α | N/m | Rs | k | α | N/m | Rs |
| AV | 13.5 | 56,878 | 12.7 | 237,759 | 23.5 | 543,055 | 27.7 | 749,816 | ||||||||
| 1.0 | 0.2 | 1.0 | 0.9 | 1.0 | 1.5 | 1.0 | 1.6 | |||||||||
| OMC | 13.7 | 73,457 | 13.0 | 352,883 | 24.5 | 389,656 | 28.9 | 478,847 | ||||||||
| 1.1 | 0.9 | 1.1 | 3.4 | 1.1 | 2.0 | 1.0 | 2.6 | |||||||||
| OCT | 14.3 | 79,834 | 14.0 | 405,038 | 26.0 | 416,811 | 30.8 | 603,501 | ||||||||
| - | - | - | - | - | - | - | - | |||||||||
| EHT | 25.0 | 357,860 | 25.5 | 1,500,064 | 42.4 | 1,174,803 | 55.5 | 1,805,575 | ||||||||
| - | - | - | - | - | - | - | - | |||||||||
| BEMT | 28.7 | 316,880 | 26.8 | 881,338 | 47.3 | 928,022 | 62.8 | 837,478 | ||||||||
| UV Filters | Linear Range (µg/mL) | Determination Coefficient (r2) | LOD (ng/mL) | Interday (%, n = 6) a | Intraday (%, n = 6) a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q1 | Q2 | Q3 | ||||||||||
| RSD | RE | RSD | RE | RSD | RE | RSD | RE | RSD | RE | RSD | RE | ||||
| AV | 0.05–5 | 0.999 | 15 | 1.9 | 3.3 | 2.8 | −4.0 | 1.2 | −1.9 | 1.6 | 0.2 | 0.4 | 1.8 | 0.8 | 2.2 |
| OMC | 0.05–5 | 0.998 | 15 | 4.9 | 0.2 | 4.2 | −2.7 | 3.9 | 1.1 | 1.8 | 0.6 | 1.6 | 0.2 | 1.4 | 0.3 |
| OCT | 0.1–10 | 0.999 | 15 | 2.8 | −0.8 | 3.1 | −2.6 | 1.2 | −1.5 | 1.4 | 0.5 | 0.8 | 2.5 | 0.3 | 0.8 |
| EHT | 0.05–5 | 0.999 | 15 | 3.4 | −1.3 | 3.0 | −2.0 | 1.5 | −1.6 | 1.5 | −2.0 | 1.2 | −1.0 | 0.3 | −0.4 |
| BEMT | 0.05–5 | 0.999 | 15 | 3.9 | −1.8 | 3.1 | −2.1 | 1.2 | −1.5 | 2.2 | −1.3 | 0.8 | −1.7 | 1.4 | −0.2 |
| Instrumental Method | Pretreatment Method | Sample | Solvent | Linear Range (ng/mL) | LOD (ng/mL) | Analyte | Ref. |
|---|---|---|---|---|---|---|---|
| GC–MS | SPME | Fish tissue | 1000–7000 a | 5–25 a | OMC, OCT | [4] | |
| HPLC–MS/MS | PLE | Cosmetics | MeOH | 1–1000 | 10–31 a | AV, OMC, OCT | [5] |
| HPLC–MS/MS | UAE–dSPE | Human placenta tissue | ACN | 0.3–15 a | 0.1 a | OMC, OCT | [6] |
| HPLC–MS/MS | QuEChERS–reverse SPE | Aquatic invertebrates | ACN | 0.1–25 | 2.0–3.3 a | OMC, OCT | [7] |
| HPLC–DAD | SLE | Porcrine skin | DMF, EtOH | 10,000–50,000 | 280–1400 | BEMT, EHT | [8] |
| HPLC–DAD | dilution | Cosmetics | MeOH, EA | 1800–250,000 | 32–67 | AV, OCT | [9] |
| HPLC–DAD | US–VA–DLLME | Cosmetics, extracts of the human stratum corneum | MeOH, anisole | 50–5000; 100–10,000 b | 15 | AV, OMC, OCT, EHT, BEMT | This study |
| UV Filters | o/w a | w/o a | ||||
|---|---|---|---|---|---|---|
| Found (μg/mL) | RSD (%) | Recovery (%) | Found (μg/mL) | RSD (%) | Recovery (%) | |
| AV | 2.2 | 2.4 | 88.1 | 2.2 | 0.8 | 87.8 |
| OMC | 2.5 | 2.3 | 100.2 | 2.5 | 2.6 | 100.2 |
| OCT | 2.4 | 2.4 | 95.7 | 2.6 | 2.6 | 104.7 |
| EHT | 2.5 | 3.3 | 99.4 | 2.5 | 3.7 | 99.4 |
| BEMT | 2.5 | 5.4 | 100.6 | 2.5 | 2.3 | 98.4 |
| UV Filters | Single | Twice | ||||||
|---|---|---|---|---|---|---|---|---|
| Cotton | S.C. | Cotton | S.C. | |||||
| Mean [Range, μg] | RSD (%) | Mean [Range, μg] | RSD (%) | Mean [Range, μg] | RSD (%) | Mean [Range, μg] | RSD (%) | |
| AV | 18.5 [8.6–37.4] | 46.0 | 14.7 [6.1–32.2] | 57.3 | 39.4 [22.2–49.9] | 20.5 | 23.6 [12.4–53.9] | 52.8 |
| OMC | 18.0 [8.3–32.9] | 48.8 | 16.4 [7.4–27.9] | 41.0 | 42.2 [21.8–61.2] | 26.6 | 30.0 [16.1–47.8] | 46.2 |
| OCT | 17.2 [8.9–29.6] | 38.0 | 14.1 [6.0–26.1] | 49.6 | 38.3 [22.8–50.6] | 21.2 | 23.6 [12.0–53.9] | 53.6 |
| EHT | 5.8 [2.6–12.9] | 61.1 | 13.4 [5.3–27.6] | 51.3 | 18.2 [5.3–34.6] | 57.1 | 22.4 [10.6–43.5] | 44.7 |
| BEMT | 5.6 [2.6–12.0] | 58.7 | 13.3 [5.7–28.1] | 52.4 | 18.2 [5.5–33.2] | 56.6 | 21.9 [11.1–41.5] | 42.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.-Y.; Su, Y.-L.; Weng, J.-R.; Lin, Y.-C.; Feng, C.-H. Ultrasound–Vortex-Assisted Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Diode Array Detection for Determining UV Filters in Cosmetics and the Human Stratum Corneum. Molecules 2020, 25, 4642. https://doi.org/10.3390/molecules25204642
Liao F-Y, Su Y-L, Weng J-R, Lin Y-C, Feng C-H. Ultrasound–Vortex-Assisted Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Diode Array Detection for Determining UV Filters in Cosmetics and the Human Stratum Corneum. Molecules. 2020; 25(20):4642. https://doi.org/10.3390/molecules25204642
Chicago/Turabian StyleLiao, Fang-Yi, Yu-Lin Su, Jing-Ru Weng, Ying-Chi Lin, and Chia-Hsien Feng. 2020. "Ultrasound–Vortex-Assisted Dispersive Liquid–Liquid Microextraction Combined with High Performance Liquid Chromatography–Diode Array Detection for Determining UV Filters in Cosmetics and the Human Stratum Corneum" Molecules 25, no. 20: 4642. https://doi.org/10.3390/molecules25204642