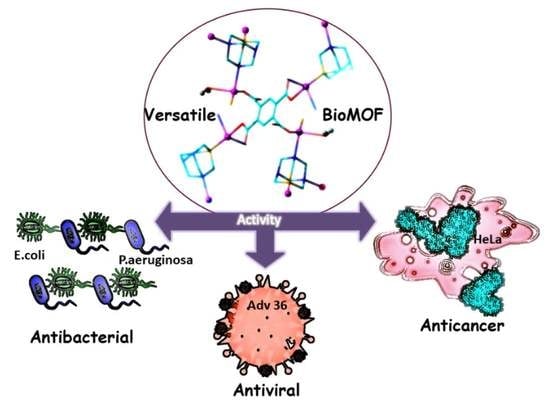
Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Procedure and Characterization
2.2. Crystal Structure
2.3. Antibacterial and Antifungal Properties
2.4. Antiviral Activity
2.5. Cytotoxic Properties on Normal and Cancer Cell Lines
3. Experimental
3.1. Materials and Methods
3.2. Antibacterial and Antifungal Activity Studies
3.3. Cell Cultures
3.4. Cytotoxicity Assay
3.5. Virucidal Activity According to PN-EN 14476
3.6. Antiviral Assay
3.7. Synthesis and Characterization of BioMOF 1
3.8. X-ray Crystallography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial applications of metal–organic frameworks and their composites. Comp. Rev. Food Sci. Food Saf. 2020, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Coelho, C.; Casadevall, A. Cryptococcal therapies and drug targets: The old, the new and the promising. Cell Microbiol. 2016, 18, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Adalja, A.; Inglesby, T. Broad-Spectrum Antiviral Agents: A Crucial Pandemic Tool. Expert. Rev. Anti-infect. Ther. 2019, 17, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Cao, P.; Wu, X.; Zhang, W.; Zhao, L.; Sun, W.; Tang, Z. Killing Oral Bacteria Using Metal−Organic Frameworks. Ind. Eng. Chem. Res. 2020, 59, 1559–1567. [Google Scholar] [CrossRef]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactivecomponents. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zoa, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent advances in the medical use of silver complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef]
- Melaiye, A.; Younges, J.W. Silver and its application as an antimicrobial agent. Expert Opin. Ther. Patents 2005, 15, 125–130. [Google Scholar] [CrossRef]
- Seifullina, I.I.; Martsinka, E.È.; Gridina, T.L.; Chebanenko, E.A.; Mudrik, L.M.; Fedchuk, A.S. Antiviral properties of the new coordination compound silver bis(citrato)germanate. Pharm. Chem. J. 2019, 53, 318–321. [Google Scholar] [CrossRef]
- Sánchez, O.; González, S.; Higuera-Padilla, Á.R.; León, Y.; Coll, D.; Fernández, M.; Taylor, P.; Urdanibia, I.; Rangel, H.R.; Ortega, J.T.; et al. Remarkable in vitro anti-HIV activity of new silver(I)– and gold(I)–N-heterocyclic carbene complexes. Synthesis, DNA binding and biological evaluation. Polyhedron 2016, 101, 14–23. [Google Scholar] [CrossRef]
- Kyros, I.; Kourkoumelis, N.; Kubicki, M.; Male, I.; Hursthouse, M.B.; Verginadis, I.I.; Gouma, E.; Karkabounes, S.; Charalabopulos, K.; Hadjikakou, S.K. Structural Properties, Cytotoxicity, and Anti-Inflammatory Activity of Silver(I) Complexes with tris(p-tolyl)Phosphine and 5-Chloro-2-Mercaptobenzothiazole. Bioinorg. Chem. Appl. 2010, 386860–386872. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Smolenski, P.; da Silva, M.F.C.; Florek, M.; Krol, J.; Staroniewicz, Z.; Pombeiro, A.J.; Kirillov, A.M. New Silver BioMOFs driven by 1,3,5-Triaza-7-phosphaadamantane-7-sulfide (PTA=S): Self-assembly Synthesis, Topological Analysis and Antimicrobial Activity. Cryst. Growth Des. 2014, 14, 5408–5417. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Wieczorek, S.W.; Lis, A.; da Silva, M.F.C.G.; Florek, M.; Król, J.; Staroniewicz, Z.; Smoleński, P.; Pombeiro, A.J.L. 1,3,5-Triaza-7-phosphaadamantane-7-oxide (PTA=O): New diamondoid building block for design of three-dimensional metal-organic frameworks. Cryst. Growth Des. 2011, 11, 2711–2716. [Google Scholar] [CrossRef]
- Jaros, S.W.; Smoleński, P.; da Silva, M.F.C.G.; Florek, M.; Król, J.; Staroniewicz, Z.; Pombeiro, A.J.L.; Kirillov, A.M. New silver BioMOFs driven by 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA=S): Synthesis, topological analysis and antimicrobial activity. CrystEngComm 2013, 15, 8060–8064. [Google Scholar] [CrossRef]
- Smoleński, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucc, M.; Vitali, L.A.; Petrelli, D.; Kochel, A.; Kirillov, A.M. New water-soluble polypyridine silver(I) derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA) with significant antimicrobial and antiproliferative activities. Dalton Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Wieczorek, S.W.; da Silva, M.F.C.G.; Sokolnicki, J.; Smoleński, P.; Pombeiro, A.J.L. Crystal engineering with 1,3,5-triaza-7-phosphaadamantane (PTA): First PTA-driven 3D metal-organic frameworks. CrystEngComm 2011, 13, 6329–6333. [Google Scholar] [CrossRef]
- Jaros, S.W.; da Silva, M.F.C.G.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-triaza-7-phosphaadamantane coordination polymers driven by substituted glutarate and malonate building blocks: self-assembly synthesis, structural features, and antimicrobial properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef]
- Jaros, S.W.; da Silva, M.F.C.G.; Król, J.; Oliveira, M.C.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Bioactive silver—organic networks assembled from 1,3,5-triaza-7-phosphaadamantane and flexible cyclohexanecarboxylate blocks. Inorg. Chem. 2016, 55, 1486–1496. [Google Scholar] [CrossRef]
- Pettinari, C.P.; Marchetti, F.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Petrelli, D.; Vitali, L.; da Silva, M.F.C.G.; Martins, L.M.D.R.S.; Smoleński, P.; et al. Synthesis, antimicrobial and antiproliferative activity of novel silver(I) tris(pyrazolyl)methanesulfonate and 1,3,5-triaza-7-phosphadamantane complexes. Inorg. Chem. 2011, 50, 11173–11183. [Google Scholar] [CrossRef] [PubMed]
- Smoleński, P.; Pettinari, C.; Marchetti, F.; Guedes da Silva, M.F.C.; Lupidi, G.; Patzmay, G.V.B.; Petrelli, D.; Vitali, L.; Pombeiro, A.J.L. Syntheses, Structure and Antimicrobial Activity of New Remarkably Light-stable and Water-soluble Tris(pyrazolyl)methanesulfonate Silver(I) Derivatives of N-methyl-1,3,5-Triaza-7-phosphaadamantane salt - [mPTA]BF4. Inorg. Chem. 2015, 54, 434–440. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA): Transition metal complexes and related catalytic, medicinal and photoluminescent applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Bravo, J.; Bolaño, J.; Gonsalvi, L.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) and derivatives. Part II. The quwst for tailored ligands, complexes and related applications. Coord. Chem. Rev. 2009, 254, 555–607. [Google Scholar] [CrossRef]
- Guerriero, A.; Peruzzini, M.; Gonsalvi, L. Coordination chemistry of 1,3,5-triaza-7-phosphatricyclo [3.3.1.1]decane (PTA) and derivatives. Part III. Variations on theme: Novel architectures, materials and applications. Coord. Chem. Rev. 2018, 355, 328–361. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Kirillov, A.M.; Smoleński, P.; Pombeiro, A.J.L. Engineering coordination and supramolecular copper-organic networks by aqueous medium self-assembly with 1,3,5-triaza-7-phosphaadamantane (PTA). Cryst. Growth Des. 2009, 9, 3006–3010. [Google Scholar] [CrossRef]
- Jaremko, Ł.; Kirillov, A.M.; Smoleński, P.; Lis, T.; Pombeiro, A.J.L. Extending the coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) to cobalt centres: first examples of Co-PTA complexes and of a metal complex with the PTA oxide ligand. Inorg. Chem. 2008, 47, 2922–2924. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Smoleński, P.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. The first copper complexes bearing the 1,3,5-triaza-7-phosphaadamantane (PTA) ligand. Eur. J. Inorg. Chem. 2007, 2686–2692. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Groenewold, G.S.; Jong, W.A.; Oomens, J.; Van Stipdonk, M.J. Variable Denticity in Carboxylate Binding tothe Uranyl Coordination Complexes. J. Am. Soc. Mass. Spectrom. 2010, 21, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; da Silva, M.F.C.G.; Kopylovich, M.N.; da Silva, J.J.R.F.; Pombeiro, A.J.L. 3D hydrogen bonded metal-organic frameworks constructed from [M(H2O)6][M′(dipicolinate)2] · mH2O (M/M′ = Zn/Ni or Ni/Ni). Identification of intercalated acyclic (H2O)6/(H2O)10 clusters. Inorg. Chim. Acta 2008, 361, 1728–1731. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Tronova, E.A.; Haukka, M.; Kirillov, A.M.; Kukushkin, V.Y.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Identification of Hexameric Water and Hybrid Water–Chloride Clusters Intercalated in the Crystal Hosts of (Imidoylamidine)nickel(II) Complexes. Eur. J. Inorg. Chem. 2007, 4621–4627. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr CompComm Newsl. 2006, 7, 4. [Google Scholar]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Therap. 2015, 40, 277–283. [Google Scholar]
- No time to Wait: Securing the future from drug-resistant infections; Report to the Secretary-General; United Nations: Geneva, Switzerland, 23 July 2019.
- Jaros, S.W.; Haukka, M.; Florek, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kirillov, A.M.; Smoleński, P. New microbe killers: Self-assembled silver(I) coordination polymers driven by a cagelike aminophosphine. Materials 2019, 12, 3353. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Shimizu, Y.; Kumagai, K. Specific Inactivation of Herpes Simplex Virus by Silver Nitrate at Low Concentrations and Biological Activities of the Inactivated Virus. Antimicrob. Agents Chemother. 1976, 10, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef]
- Lenaerts, L.; Naesens, L. Antiviral therapy for adenovirus infections. Antivir. Res. 2006, 71, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ponterio, E.; Gnessi, L. Adenovirus 36 and Obesity: An Overview. Viruses 2015, 7, 3719–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinchington, P.R.; Romanowski, E.G.; Gordon, Y.J. Prospects for adenovirus antiviral. J. Antimicrob. Chemother. 2005, 55, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Koa, G.P. Antiviral Properties of Silver Nanoparticles on a Magnetic Hybrid Colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zheng, Y.; Yin, J.; Li, X.; Zheng, C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods 2013, 193, 470–477. [Google Scholar] [CrossRef] [PubMed]
- EN 14476. Chemical Disinfectants and Antiseptics–Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area–Test Method and Requirements (Phase2/Step 1); European Committee for Standarization: Brussels, Belgium, 2013. [Google Scholar]
- Qi, Y.; Pradipta, A.R.; Li, M.; Zhao, X.; Lu, L.; Fu, X.; Wei, J.; Hsung, R.P.; Tanaka, K.; Zhou, L. Cinchonine induces apoptosis of HeLa and A549 cells through targeting TRAF6. J. Exp. Clin. Cancer Res. 2017, 36, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Comparative cytotoxicity evaluation of differentsize gold nanoparticles in human dermal fibroblasts. J. Exp. Nanosci. 2015, 10, 1401–1417. [Google Scholar] [CrossRef] [Green Version]
- Jaros, S.W.; Śliwińska-Hill, U.; Białońska, A.; Nesterov, D.S.; Kuropka, P.; Sokolnicki, J.; Bażanów, B.; Smoleński, P. Light-stable polypyridine silver(I) complexes of 1,3,5-triaza-7-phosphaadamantane (PTA) and 1,3,5-triaza-7-phosphaadamantane-7-sulfide (PTA=S): Significant antiproliferative activity of representative examples in aqueous media. Dalton Trans. 2019, 48, 11235–11249. [Google Scholar] [CrossRef]
- Sgarbossa, P.; Śliwińska-Hill, U.; Fátima, M.; da Silva, M.F.C.G.; Bażanów, B.; Pawlak, A.; Jackulak, N.; Poradowski, D.; Pombeiro, A.J.L.; Smoleński, P. Pentafluorophenyl Platinum(II) Complexes of PTA and its N-Allyl and N-Benzyl Derivatives: Synthesis, Characterization and Biological Activity. Materials 2019, 12, 3907. [Google Scholar] [CrossRef] [Green Version]
- Daigle, D.J.; Pepperman, A.B., Jr.; Vail, S.L. Synthesis of a monophosphorus analog of hexamethylenetetramine. J. Heterocycl. Chem. 1974, 11, 407–408. [Google Scholar] [CrossRef]
- Daigle, D.J. 1,3,5-Triaza-7-Phosphatricyclo[3.3.1.13,7]Decane and Derivatives. Inorg. Synth. 1998, 32, 40–45. [Google Scholar] [CrossRef]
- Grove, D.C.; Randall, W.A. Assay Methods of Antibiotic. A Laboratory Manual; Medical Encyclopedia: New York, NJ, USA, 1955. [Google Scholar]
- Lorian, V. Antibiotics in Laboratory Medicine, 2nd ed.; Williams & Wilkins: Philadelphia, PA, USA, 1986; p. 93. [Google Scholar]
- Balouini, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C. Method for the Determination of Broth Dilution of Antifungal agents for Fermentative Yeasts; Document, E.DEF 7.3; EUCAST: Copenhagen, Denmark, December 2015. [Google Scholar]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, Oxfordshire, England, 2014. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond; Version, 4.0; Crystal, Molecular Structure Visualization; Crystal Impact, K. Brandenburg and H. Putz Gbr: Bonn, Germany, 2009. [Google Scholar]
Sample Availability: Samples of the compound 1 are available from the authors. |


| MIC [µg·mL−1] | Normalized MIC [nmol·mL−1] a | ||||
|---|---|---|---|---|---|
| Entry | Strains | 1 | AgNO3 b | 1 | AgNO3 b |
| 1 | P. aeruginosa | 5 | 9 | 14 | 53 |
| 2 | E. coli | 5 | 9 | 14 | 53 |
| 3 | S. aureus | 8 | 20 | 22 | 118 |
| 4 | C. albicans | 30 | 40 | 83 | 236 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaros, S.W.; Król, J.; Bażanów, B.; Poradowski, D.; Chrószcz, A.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid. Molecules 2020, 25, 2119. https://doi.org/10.3390/molecules25092119
Jaros SW, Król J, Bażanów B, Poradowski D, Chrószcz A, Nesterov DS, Kirillov AM, Smoleński P. Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid. Molecules. 2020; 25(9):2119. https://doi.org/10.3390/molecules25092119
Chicago/Turabian StyleJaros, Sabina W., Jarosław Król, Barbara Bażanów, Dominik Poradowski, Aleksander Chrószcz, Dmytro S. Nesterov, Alexander M. Kirillov, and Piotr Smoleński. 2020. "Antiviral, Antibacterial, Antifungal, and Cytotoxic Silver(I) BioMOF Assembled from 1,3,5-Triaza-7-Phoshaadamantane and Pyromellitic Acid" Molecules 25, no. 9: 2119. https://doi.org/10.3390/molecules25092119