Novel Steroidal 5α,8α-Endoperoxide Derivatives with Semicarbazone/Thiosemicarbazone Side-chain as Apoptotic Inducers through an Intrinsic Apoptosis Pathway: Design, Synthesis and Biological Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxic Activity
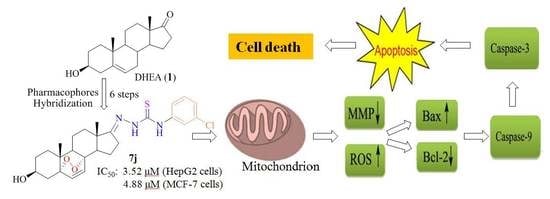
2.2.2. Compound 7j Induces Apoptosis in HepG2 Cells
2.2.3. Compound 7j Induces Mitochondrial Membrane Potential (MMP) Loss in HepG2 Cells
2.2.4. Compound 7j Induces Oxidative Stress in HepG2 Cells
2.2.5. Compound 7j Induces Apoptosis Via the Activation of Caspases and Regulated Apoptosis Releated Protein Expression
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of 3β-Acetoxyandrosta-5-en-17-one (2)
4.1.2. Synthesis of 3β-Acetoxyandrosta-5,7-diene-17-one (3)
4.1.3. Synthesis of 3β-Hydroxyandrosta-5,7-diene-17-one (4)
4.1.4. Synthesis of 5α,8α-Cyclicobioxygen-6-vinyl-3β-ol-DHEA(5)
4.1.5. Synthesis of 5α,8α-Cyclicobioxygen-6-vinyl-3β-ol-DHEA(6)
4.1.6. General Procedure for Synthesis of Novel Derivatives 7a–k
4.2. Biological Evaluation
4.2.1. Cell Culture
4.2.2. MTT Assay
4.2.3. Determination of Morphological Changes of Cells
4.2.4. Apoptosis Analysis by Flow Cytometry
4.2.5. Analysis of Mitochondrial Membrane Potential
4.2.6. Measurement of ROS by Flow Cytometry
4.2.7. Western Blot Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, M.M.; Qiao, Y.; Ang, E.L.; Zhao., H. Using natural products for drug discovery: The impact of the genomics era. Expert. Opin. Drug. Discov. 2017, 12, 475–487. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Yang, B.B.; Hu, L.M. Natural endoperoxides as drug lead compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef]
- Jung, M.; Kim, H.; Lee, K.; Park, M. Naturally occurring peroxides with biological activities. Mini-Rev. Med. Chem. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Yukawa, M.; Ueno, M.; Kimura, K.I.; Tsuchiya, E. 3,6-Epidioxy-1,10-bisaboladiene inhibits G1 -specific transcription through Swi4/Swi6 and Mbp1/Swi6 via the Hog1 stress pathway in yeast. FEBS J. 2014, 281, 4612–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini-Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef]
- Callaway, E.; Cyranoski, D. Anti-parasite drugs sweep Nobel prize in medicine 2015. Nature 2015, 526, 174–185. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating helminthic infections beyond schistosomiasis. Pharmacol. Res. 2018, 133, 77–100. [Google Scholar] [CrossRef]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and its derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [PubMed] [Green Version]
- Cui, J.G.; Liu, L.; Zhao, D.D.; Gan, C.F.; Huang, X.; Xiao, Q.; Qi, B.B.; Yang, L.; Huang, Y.M. Synthesis, characterization and antitumor activities of some steroidal derivatives with side chain of 17-hydrazone aromatic heterocycle. Steroids 2015, 95, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.Y.; Wei, Y.H.; Shi, L.Q.; Yang, Q.Y.; Yang, Z.W. Synthesis of novel steroid derivatives derived from dehydroepiandrosterone as potential anticancer agents. Anticancer Agents Med. Chem. 2013, 13, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, E.; Sun, X.N.; Ren, J.L.; Fang, Y.; Zhang, B.L.; Yu, D.Q.; Liu, H.M. Facile synthesis of novel D-ring modified steroidal dienamides via rearrangement of 2H-pyrans. Steroids 2013, 78, 494–499. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yang, J.; Guo, L.; Wang, X.; Song, B.; Dong, W.; Wang, W. Novel diosgenin derivatives containing 1,3,4-oxadiazole/thiadiazole moieties as potential antitumor agents: Design, synthesis and cytotoxic evaluation. Eur. J. Med. Chem. 2020, 186, 111897. [Google Scholar] [CrossRef]
- Hanson, J.R. Steroids: Partial synthesis in medicinal chemistry. Nat. Prod. Rep. 2010, 27, 887–889. [Google Scholar] [CrossRef]
- Jeong, Y.U.; Park, Y.J. Ergosterol peroxide from the medicinal mushroom Ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. Int. J. Mol. Sci. 2020, 21, E460. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Shi, W.; Liu, X.; Zhao, X.; Zhang, Z. Anticancer action and mechanism of ergosterol peroxide from paecilomyces cicadae fermentation broth. Int. J. Mol. Sci. 2018, 19, E3935. [Google Scholar] [CrossRef] [Green Version]
- Nowak, R.; Drozd, M.; Mendyk, E.; Lemieszek, M.; Krakowiak, O.; Kisiel, W.; Rzeski, W.; Szewczyk, K. A new method for the isolation of ergosterol and peroxy ergosterol as active compounds of hygrophoropsis aurantiaca and in vitro antiproliferative activity of isolated ergosterol peroxide. Molecules 2016, 21, E946. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.P.; Xie, Y.Z.; Deng, Z.; Li, X.M.; Yang, W.; Jiao, C.W.; Fang, L.; Li, S.Z.; Pan, H.H.; Yee, A.J.; et al. Ergosterol peroxide isolated from Ganoderma lucidum abolishes microRNA miR-378-mediated tumor cells on chemoresistance. PLoS ONE 2012, 7, e44579. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, Q.P.; Bu, M.; Hu, L.M.; Du, W.W.; Jiao, C.W.; Pan, H.H.; Sdiri, M.; Wu, N.; Xie, Y.Z.; et al. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 2016, 7, 33948–33959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, M.; Cao, T.T.; Li, H.X.; Guo, M.Z.; Yang, B.B.; Zhou, Y.; Zhang, N.; Zeng, C.C.; Hu, L.M. Synthesis and biological evaluation of novel steroidal 5α,8α-endoperoxide derivatives with aliphatic side-chain as potential anticancer agents. Steroids 2017, 124, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Cao, T.T.; Li, H.X.; Guo, M.Z.; Yang, B.B.; Zeng, C.C.; Hu, L.M. Synthesis of 5α,8α-ergosterol peroxide 3-carbamate derivatives and a fluorescent mitochondria-targeting conjugate for enhanced anticancer activities. ChemMedChem 2017, 12, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Li, H.; Wang, H.; Wang, J.; Lin, Y.; Ma, Y. Synthesis of ergosterol peroxide conjugates as mitochondria targeting probes for enhanced anticancer activity. Molecules 2019, 24, E3307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, M.; Cao, T.; Li, H.; Guo, M.; Yang, B.B.; Zeng, C.; Zhou, Y.; Zhang, N.; Hu, L. Synthesis and biological evaluation of novel steroidal 5α,8α-epidioxyandrost-6-ene-3β-ol-17-(O-phenylacetamide)oxime derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 3856–3861. [Google Scholar] [CrossRef]
- Wang, H.J.; Bu, M.; Wang, J.; Liu, L.; Zhang, S. Synthesis and biological evaluation of novel steroidal 5α, 8α-endoperoxide steroidal derivatives with aromatic hydrazone side chain as potential anticancer agents. Russ. J. Bioorg. Chem. 2019, 45, 585–590. [Google Scholar] [CrossRef]
- Li, H.L.; Wang, H.J.; Wang, J.; Lin, Y.; Ma, Y.; Bu, M. Design, synthesis and biological evaluation of novel 5α, 8α-endoperoxide steroidal derivatives with hybrid side chain as anticancer agents. Steroids 2020, 153, 108471. [Google Scholar] [CrossRef]
- Qazi, S.U.; Rahman, S.U.; Awan, A.N.; Al-Rashida, M.; Alharthy, R.D.; Asari, A.; Hameed, A.; Iqbal, J. Semicarbazone derivatives as urease inhibitors: Synthesis, biological evaluation, molecular docking studies and in-silico ADME evaluation. Bioorg. Chem. 2018, 79, 19–26. [Google Scholar] [CrossRef]
- Sinniah, S.K.; Tan, K.W.; Ng, S.W.; Sim, K.S. Thiosemicarbazone derivative induces in vitro apoptosis in metastatic PC-3 cells via activation of mitochondrial pathway. Anticancer Agents Med. Chem. 2017, 17, 741–753. [Google Scholar] [CrossRef]
- Xu, H.; Su, X.; Liu, X.Q.; Zhang, K.P.; Hou, Z.; Guo, C. Design, synthesis and biological evaluation of novel semicarbazone-selenochroman-4-ones hybrids as potent antifungal agents. Bioorg. Med. Chem. Lett. 2019, 29, 126726. [Google Scholar] [CrossRef]
- Cavalcanti de Queiroz, A.; Alves, M.A.; Barreiro, E.J.; Lima, L.M.; Alexandre-Moreira, M.S. Semicarbazone derivatives as promising therapeutic alternatives in leishmaniasis. Exp. Parasitol. 2019, 201, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Demoro, B.; Bento-Oliveira, A.; Marques, F.; Costa Pessoa, J.; Otero, L.; Gambino, D.; F M de Almeida, R.; Tomaz, A.I. Interaction with blood proteins of a ruthenium (II) nitrofuryl semicarbazone complex: Effect on the antitumoral activity. Molecules 2019, 24, E2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Ni, X.; Gao, Y.; Huang, K.; Wang, Y.; Liu, J.; Gong, G. Semicarbazone derivatives bearing phenyl moiety: Synthesis, anticancer activity, cell cycle, apoptosis-inducing and metabolic stability study. Chem. Pharm. Bull. 2019, 67, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Poon, R.; Sahni, S.; Anjum, R.; Hibbs, D.; Lin, H.Y.; Bernhardt, P.V.; Kalinowski, D.S.; Richardson, D.R. A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 612–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and bioactivity of thiosemicarbazones containing adamantane skeletons. Molecules 2020, 25, E324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Halogenated aromatic thiosemicarbazones as potent inhibitors of tyrosinase and melanogenesis. Bioorg. Chem. 2020, 94, 103419. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Asiri, A.M. Multi-step synthesis, spectroscopic studies of biological active steroidal thiosemicarbazones and their palladium (II) complex as macromolecules. Int. J. Biol.Macromol. 2018, 107, 105–111. [Google Scholar] [CrossRef]
- Jabeen, M.; Choudhry, M.I.; Miana, G.A.; Rahman, K.M.; Rashid, U.; Khan, H.U.; Sadiq, A. Synthesis, pharmacological evaluation and docking studies of progesterone and testosterone derivatives as anticancer agents [J]. Steroids 2018, 136, 22–31. [Google Scholar] [CrossRef]
- Yadav, P.; Lal, K.; Kumar, A.; Guru, S.K.; Jaglan, S.; Bhushan, S. Green synthesis and anticancer potential of chalcone linked-1,2,3-triazoles. Eur. J. Med. Chem. 2017, 126, 944–953. [Google Scholar] [CrossRef]
- Weidner, C.; Rousseau, M.; Plauth, A.; Wowro, S.J.; Fischer, C.; Abdel-Aziz, H.; Sauer, S. Iberisamara extract induces intracellular formation of reactive oxygen species and inhibits colon cancer. PLoS ONE 2016, 11, e0152398. [Google Scholar] [CrossRef]
- Swanepoel, B.; Nitulescu, G.M.; Olaru, O.T.; Venables, L.; van de Venter, M. Anti-cancer activity of a 5-aminopyrazole derivative lead compound (BC-7) and potential synergistic cytotoxicity with cisplatin against human cervical cancer cells. Int. J. Mol. Sci. 2019, 20, E5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wang, M.; Wang, C.; Hu, W.; You, Q.; Yang, Y.; Yu, C.; Liao, Z.; Gou, S.; Wang, H. Dual-targeting antitumor conjugates derived from platinum (IV) prodrugs and microtubule inhibitor CA-4 significantly exhibited potent ability to overcome cisplatin resistance. Bioorg. Chem. 2019, 92, 103236. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Siveen, K.S.; Kuttikrishnan, S.; Jochebeth, A.; Ali, T.A.; Elareer, N.R.; Iskandarani, A.; Quaiyoom Khan, A.; Merhi, M.; Dermime, S.; et al. Greensporone A, a fungal secondary metabolite suppressed constitutively activated AKT via ROS generation and induced apoptosis in leukemic cell lines. Biomolecules 2019, 9, E126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Zhang, C.; Liu, B.; Shu, Y.; Du, T.; Shu, D.; Wang, K.; Dai, F.; Liu, Y.; Li, C.; et al. Regression of gastric cancer by systemic injection of RNA nanoparticles carrying both ligand and siRNA. Sci. Rep. 2015, 5, 10726. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7a–k are available from the authors. |










| Compound | X | R | IC50 (μM) a | |||
|---|---|---|---|---|---|---|
| HepG2 | HCT-116 | MCF-7 | A549 | |||
| 7a | O | 4-Cl | 8.65 ±0.72 | 32.59 ± 2.18 | 19.31 ± 1.08 | 21.43 ± 1.12 |
| 7b | O | 3-CF3 | 5.73 ± 0.78 | 10.48 ± 0.59 | 8.62 ± 0.71 | 18.04 ± 0.93 |
| 7c | O | 4-OCH3 | >60 | >60 | 52.32 ± 2.14 | >60 |
| 7d | O | 4-CH3 | 37.50 ± 2.26 | >60 | >60 | 47.33 ± 2.17 |
| 7e | O | 4-CF3 | 7.51 ± 0.77 | 26.44 ± 1.43 | 14.90 ± 0.90 | 21.88 ± 1.21 |
| 7f | O | 3-CF3-4-Cl | 4.34 ± 0.41 | 12.78 ± 1.42 | 7.37 ± 0.42 | 6.03 ± 0.81 |
| 7g | O | 4-CN | 6.06 ± 0.69 | 14.07 ± 1.58 | 8.98 ± 0.70 | 16.93 ± 1.77 |
| 7h | S | 4-Cl | 5.44 ± 0.42 | 15.41 ± 1.63 | 7.66 ± 0.49 | 11.33 ± 0.69 |
| 7i | S | 4-OCH3 | 14.35± 0.82 | >60 | 26.73 ± 1.75 | >60 |
| 7j | S | 3-Cl | 3.52± 0.35 | 9.79 ± 0.55 | 4.88 ± 0.42 | 7.70 ± 0.62 |
| 7k | S | 4-CH3 | 19.07 ± 1.09 | 48.36 ± 4.43 | 25.73 ± 1.52 | >60 |
| EP | - | - | 21.35± 1.13 | 26.42± 1.32 | 16.04 ± 0.81 | 19.48 ± 1.16 |
| Mitomycin | - | - | 29.36 ± 1.14 | 10.52± 0.87 | 16.56± 1.09 | 12.47 ± 1.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, H.; Wang, J.; Liu, L.; Zhang, S.; Bu, M. Novel Steroidal 5α,8α-Endoperoxide Derivatives with Semicarbazone/Thiosemicarbazone Side-chain as Apoptotic Inducers through an Intrinsic Apoptosis Pathway: Design, Synthesis and Biological Studies. Molecules 2020, 25, 1209. https://doi.org/10.3390/molecules25051209
Ma L, Wang H, Wang J, Liu L, Zhang S, Bu M. Novel Steroidal 5α,8α-Endoperoxide Derivatives with Semicarbazone/Thiosemicarbazone Side-chain as Apoptotic Inducers through an Intrinsic Apoptosis Pathway: Design, Synthesis and Biological Studies. Molecules. 2020; 25(5):1209. https://doi.org/10.3390/molecules25051209
Chicago/Turabian StyleMa, Liwei, Haijun Wang, Jing Wang, Lei Liu, Song Zhang, and Ming Bu. 2020. "Novel Steroidal 5α,8α-Endoperoxide Derivatives with Semicarbazone/Thiosemicarbazone Side-chain as Apoptotic Inducers through an Intrinsic Apoptosis Pathway: Design, Synthesis and Biological Studies" Molecules 25, no. 5: 1209. https://doi.org/10.3390/molecules25051209