Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls
Abstract
:1. Introduction
2. Excited-State Quenching, Antioxidant, and Pro-Vitamin A Activity of Carotenoids
3. Carotenoids and Carcinogenesis
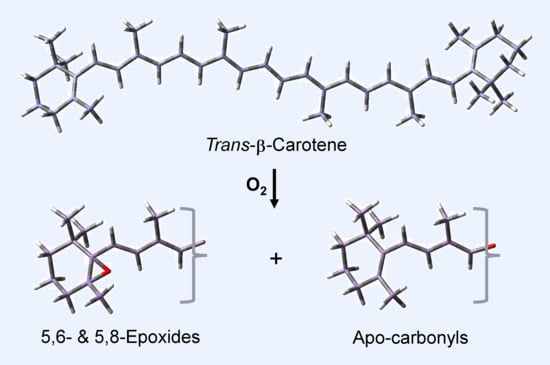
4. Oxidative Degradation of Carotenoids
5. Products from Oxidations of Carotenes and Xanthophylls
6. Mechanisms of the Oxidation of Carotenes and Xanthophylls by Molecular Oxygen
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Amaya, D.B. Update on natural food pigments–A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Munzel, K. Carotenoids in pharmaceutical and cosmetic products. In Carotenoids as colorants and vitamin A precursors; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 745–754. [Google Scholar]
- Moore, T. Vitamin A and Carotene: The absence of the liver oil vitamin A from carotene. VI. The conversion of carotene to vitamin A in vivo. Biochem. J. 1930, 24, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Mattson, F.H.; Mehl, J.W.; Deuel, H. Studies on carotenoid mechanism. 7. The site of conversion of carotene to vitamin A in the rat. Arch. Biochem. 1947, 15, 65–73. [Google Scholar] [PubMed]
- Glover, J.; Goodwin, T.W.; Morton, R.A. Studies in Vitamin. 8. Conversion of β-carotene into vitamin A in the intestine of the rat. Biochem. J. 1948, 43, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.Y.; Ganguly, J.; Kon, S.K. The conversion of β-carotene to vitamin A in the intestine. Br. J. Nutr. 1949, 3, 50–78. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.A.; Hayaishi, O. The enzymatic conversion of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. USA 1965, 54, 1364–1370. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, T.H.; Voutilainen, S.; Nyyssonen, K.; Lakka, T.A. Low serum lycopene concentration is associated with an excess incidence of acute coronary events and stroke: The Kuopio Ischaemic Heart Disease Risk Factor Study. Br. J. Nutr. 2001, 85, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary β-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef]
- Glover, J. The conversion of β-carotene to vitamin A. Vitamins and Hormones 1960, 18, 371–386. [Google Scholar] [PubMed]
- Sharma, R.V.; Mathura, S.N.; Dmitrovskii, A.A.; Das, R.S.; Ganguly, J. Studies on the metabolism of beta-carotene and apo-β-carotenoids in rats and chickens. Biochem. Biophys. Acta. 1976, 486, 183–194. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.D.; Russell, R.M.; Krinsky, N.I. Characterisation of β-Apo-13-carotenone and-Apo-14’-carotenal as enzymatic products excentric cleavage of β-carotene. Biochemistry. 1991, 30, 9829–9834. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Tang, G.; Fox, J.G.; Krinsky, N.I.; Russell, R.M. Enzymatic conversion of β-carotene into -apo-carotenals and retinoids by human, monkey, ferret and rat tissues. Arch. Biochem. Biophys. 1991, 285, 8–16. [Google Scholar] [CrossRef]
- Foote, C.S.; Denny, R.W. Chemistry of singlet oxygen. VII. Quenching by beta-carotene. J. Am. Chem. Soc. 1968, 90, 6233–6235. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Miyamoto, S.; Bechara, E.; Di Mascio, P.; Martinez, G.R. Oxidação de proteínas por oxigênio singlete: Mecanismos de dano, estratégias para detecção e implicações biológicas. Quím. Nova 2006, 29, 563–568. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases-a critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Wilson, T.; Fujimori, E.; Krinsky, N.I. Carotenoid chromophore length and protection against photosentisation. Photochem. Photobiol. 1974, 19, 217–222. [Google Scholar] [CrossRef]
- Matsushita, S.; Terao, J. Singlet oxygen-initiated photooxidation of unsaturated fatty acid esters and inhibitory effects of tocopherols and β-carotene. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Plenum: New York, NY, USA, 1980; pp. 27–44. [Google Scholar]
- Packer, J.E.; Mahood, J.S.; Mora-Arellano, V.O.; Slater, T.F.; Wilson, R.L.; Wolfenden, B.S. Free radicals and singlet oxygen scavengers: Reaction of a peroxyl-radical with β-carotene, diphenyl furan and 1,4-diazobicyclo(2,2,2)-octane. Biochem. Biophys. Res. Commun. 1981, 98, 901–906. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, D.B. Effects, Quenching Mechanisms, and Kinetics of Carotenoids in Chlorophyll-sensitized Photooxidation of Soybean Oil. J. Agric. Food Chem. 1990, 38, 1630–1634. [Google Scholar] [CrossRef]
- Jung, M.Y.; Min, D.B. Effects of Quenching Mechanisms of Carotenoids on the Photosensitized Oxidation of Soybean Oil. J. Am. Oil Chem. Soc. 1991, 68, 653–658. [Google Scholar] [CrossRef]
- Krinsky, N.I. Carotenoid protection against oxidation. Pure Appl. Chem. 1979, 51, 649–660. [Google Scholar] [CrossRef]
- Krinsky, N.I. β-Carotene: Functions, New Protective Roles for Selected Nutrients. Current Topics Nutrition Disease 1989, 22, 1–16. [Google Scholar]
- Krinsky, N.I.; Deneke, S.M. Interaction of oxygen and oxy-radicals with carotenoids. J. Natl. Cancer Inst. 1982, 69, 205–210. [Google Scholar]
- Burton, G.W.; Ingold, K.U. beta-carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Terao, J. Antioxidant activity of beta-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef]
- Faria, J.A.F.; Mukai, M.K. Use of gas chromatographic reactor to study lipid photoxidation. J. Am. Oil Chem. Soc. 1983, 60, 77–81. [Google Scholar] [CrossRef]
- Handelman, G.J.; van Kuijk, F.J.G.M.; Chatterjee, A.; Krinsky, N.I. Characterisation of products formed during the autoxidation of β-carotene. Free Radical Biol. Med. 1991, 10, 427–437. [Google Scholar] [CrossRef]
- Epstein, J.H. Effects of β-carotene on ultraviolet induced cancer formation in the hairless mouse skin. Photochem. Photobiol. 1977, 25, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M.; Krinsky, N.I. Carotenoid Dose Level and Protection against UV-B Induced Skin Tumors. Photochem. Photobiol. 1985, 42, 35–38. [Google Scholar] [CrossRef]
- Santamaria, L.; Bianchi, A.; Mobilio, G.; Santagi, G.; Ravetto, C.; Bernardo, G.; Vetere, C. Nutrition and Growth Cancer; Tryfiates, G.P., Prasad, K.N., Eds.; Allan. C. Liss 1nc: New York, NY, USA, 1988; p. 177. [Google Scholar]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.H.; Kass, E.H. Beta-carotene therapy for erythropoietic protoporphyria and other photosensitivity disease. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar]
- Temple, N.J.; Basu, T.K. Does beta-carotene prevent cancer? A critical appraisal. Nutr. Res. 1988, 8, 685–701. [Google Scholar]
- Lepage, G. Federation of American Societies for Experimental Biology. 75th Annual Meeting. Atlanta, Georgia, April 21-25, 1991. Part II. Abstracts. FASEB J. 1991, 5, A1075. [Google Scholar]
- Ames, B.N. Dietary carcinogens and anti-carcinogens: Oxygen radicals and degenerative diseases. Science 1983, 221, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Mathews-Roth, M.M. Antitumor activity of β -carotene, canthaxanthin and phytoene. Oncology 1982, 39, 33–37. [Google Scholar] [CrossRef]
- Suda, D.; Schwartz, J.; Shklaret, G. Inhibition of experimental oral carcinogenesis by topical beta carotene. Carcinogenesis 1986, 7, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Som, S.; Chatterjee, M.; Banerjee, M.R. β-Carotene inhibition of 7,12-dimethylbenz[a]anthracene-induced transformation of murine mammary cells in vitro. Carcinogenesis 1984, 5, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, T.V.; Francis, F.J. Autoxidation of carotenoids and their relative polarity. J. Food Qual. 1980, 3, 25–34. [Google Scholar] [CrossRef]
- Karrer, P.; Helfenstein, A.; Wehrli, H.; Wettstein, A. Pflanzenfarbstoffe XXV. Über die Konstitution des Lycopins und Carotins. Helv. Chim. Acta 1930, 13, 1084–1099. [Google Scholar] [CrossRef]
- Karrer, P.; Morf, R.; Schopp, K. Zur kenntnis des vitamins-A aus fischtranen II. Helv. Chim. Acta. 1931, 14, 1431–1436. [Google Scholar] [CrossRef]
- Hunter, R.F.; Williams, N.E. Chemical conversion of β-carotene into vitamin-A. J. Chem. Soc. 1945, 554–556. [Google Scholar] [CrossRef]
- Glover, J.; Redfearn, E.R. The mechanism of the transformation of beta-carotene into vitamin A. in vivo. Biochem. J. 1954, 58. [Google Scholar]
- Zechmeister, L.; Le Rosen, A.L.; Schoeder, W.A.; Polhar, A.; Pauling, L. Spectral characteristics and configuration of some stereoisomeric carotenoids including pro-lycopene and pro- β -carotene. J. Am. Chem. Soc. 1943, 65, 1940–1951. [Google Scholar] [CrossRef]
- El-Tinay, A.H.; Chichester, C. Oxidation of β -carotene. Site of initial attack, J. Org. Chem. 1970, 35, 2290–2293. [Google Scholar]
- Ganguly, J.; Sastry, P.S. Mechanism of Conversion of β-Carotene into Vitamin A–Central Cleavage versus Random Cleavage. World Rev. Nutr. Diet. 1985, 45, 198–220. [Google Scholar]
- Mordi, R.C.; Walton, J.C.; Burton, G.W.; Hughes, L.; Ingold, K.U.; Lindsay, D.A.; Moffatt, D.J. Oxidative Degradation of β-Carotene and β-Apo-8’-Carotenal. Tetrahedron 1993, 49, 911–928. [Google Scholar] [CrossRef]
- Mordi, R.C.; Walton, J.C.; Burton, G.W.; Hughes, L.; Ingold, K.U.; Lindsay, D.A. Exploratory study of β-carotene autoxidation. Tetrahedron Lett. 1991, 32, 4203–4206. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.A.; Leibler, D.C. Peroxyl radical oxidation of beta-carotene: Formation of beta-carotene epoxides. Chem. Res. Toxicol. 1991, 4, 290–295. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Rodriguez-Amaya, D.B. Formation of apo-carotenals and epoxy carotenoids from β -carotene by chemical reactions and by autoxidation in model systems and processed foods. Food Chem. 2007, 101, 563–572. [Google Scholar] [CrossRef]
- Friend, J. The coupled oxidation of β-carotene by a linoleate-lipoxidase system and by autoxidizing linoleate. Chem. Ind. 1958, 597–598. [Google Scholar]
- Von Doering, W.E.; Sarma, K. Stabilisation energy of polyethylene radicals: All trans-nonatetraenyl radical by thermal rearrangement of a semirigid{4-1-2}heptaene. Model of thermal stability of β-carotene. J. Am. Chem. Soc. 1992, 114, 6037–6043. [Google Scholar] [CrossRef]
- Mohamed, N.; Hashim, R.; Rahman, N.A.; Zain, S.M. An insight into the cleavage of β-carotene to vitamin A: A molecular mechanics study. J. Mol. Struct. (Thermo Chem) 2001, 538, 245–252. [Google Scholar] [CrossRef]
- Penicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Achir, N.; Penicaud, C.; Avallone, S.; Bohuon, P. Insight into β-carotene thermal degradation in oils with multiresponse modelling. J. Am. Oil Chem. Soc. 2011, 88, 2035–2045. [Google Scholar] [CrossRef]
- Marx, M.; Stuparic, M.; Schieber, A.; Carle, R. Effect of thermal processing on cis-trans isomerisation of β-carotene in carrot juices and carotene containing preparations. Food Chem. 2003, 83, 609–617. [Google Scholar] [CrossRef]
- Henry, L.K.; Catignani, G.; Schwartz, S. Oxidative degradation kinetics of lycopene, lutein and 9-cis and all-trans-β-carotene. J. Am. Oil Chem. Soc. 1998, 75, 823–829. [Google Scholar] [CrossRef]
- Mordi, R.C.; Walton, J.C. Identification of products from canthaxanthin oxidation. Food Chem. 2016, 197, 836–840. [Google Scholar] [CrossRef] [Green Version]
- Møller, A.H.; Jahangiri, A.; Danielsen, M.; Madsen, B.; Joernsgaard, B.; Vaerbak, S.; Hammershøj, M.; Dalsgaard, T.K. Mechanism behind the degradation of aqueous norbixin upon storage in light and dark environment. Food Chem. 2020, 310, 125967. [Google Scholar] [CrossRef]
- Mordi, R.C. Mechanism of beta-carotene degradation. Biochem. J. 1993, 292, 310–312. [Google Scholar]
- Mayo, F.R.; Miller, A.A. The oxidation of unsaturated compounds. VI. The effect of oxygen pressure on the oxidation of α-methylstyrene. J. Am. Chem. Soc. 1958, 80, 2480–2493. [Google Scholar]
- Porter, N.A.; Cudd, M.A.; Mller, R.W.; McPhail, A.T. A fixed-geometry study of the SH2 reaction on the peroxide bond. J. Am. Chem. Soc. 1980, 102, 414–416. [Google Scholar] [CrossRef]
- Porter, N.A.; Zuraw, P.J. Stereochemistry of hydroperoxide cyclization reactions. J. Org. Chem. 1984, 49, 1345–1348. [Google Scholar] [CrossRef]
- Bourgeois, M.J.; Maillard, B.; Montaudon, E. Homolytic intramolecular displacements. 12. Decomposition of ethylenic peroxides: Effect of chain length. Tetrahedron 1986, 42, 5309–5320. [Google Scholar] [CrossRef]
- Mordi, R.C.; Walton, J.C. Electron spin resonance study of free radicals generated from retinyl- and ionyl-derivatives. Chem. Phys. Lipids 1990, 54, 73–78. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. https://doi.org/10.3390/molecules25051038
Mordi RC, Ademosun OT, Ajanaku CO, Olanrewaju IO, Walton JC. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules. 2020; 25(5):1038. https://doi.org/10.3390/molecules25051038
Chicago/Turabian StyleMordi, Raphael C., Olabisi T. Ademosun, Christiana O. Ajanaku, Ifedolapo O. Olanrewaju, and John C. Walton. 2020. "Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls" Molecules 25, no. 5: 1038. https://doi.org/10.3390/molecules25051038