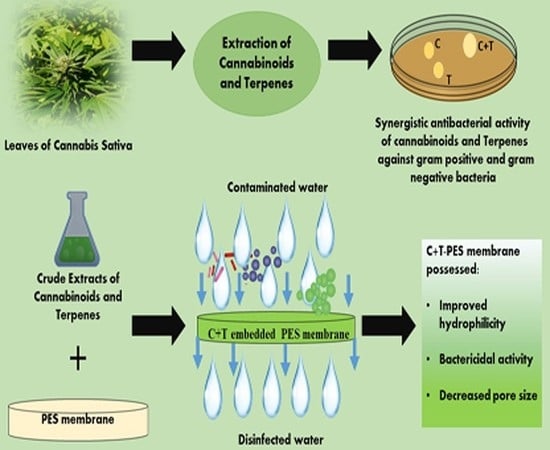
Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Cannabinoids and Terpenes from C. sativa
2.2. Synergistic Antibacterial Activity of Cannabinoids and Terpenes
2.3. Membrane Fabrication
2.4. Characterization
2.4.1. Characterization of Cannabinoids and Terpenes
2.4.2. Characterization of Pure PES and Hybrid Membranes
Fourier Transform Infrared Spectroscopy (FTIR) analysis of Hybrid Membranes
Energy-Dispersive X-ray Spectroscopy Analysis (EDX)
Morphological Analysis
2.5. Mechanical Testing
2.6. Contact Angle, Water Retention, and Surface Roughness
2.7. X-ray florescence Analysis for Surface Leaching
2.8. Membrane Flux
2.9. Quantitative Bacterial Decline Analysis of Membranes
3. Materials and Methods
3.1. Materials
3.2. Plant Collection and Extraction of Phytochemicals
3.2.1. Butanol Extraction of Cannabinoid
3.2.2. Methanol Extraction of Terpenes
3.3. Antibacterial Activity of Cannabinoids and Terpenes
3.4. Fabrication of Mixed Matrix Polymer Membrane
3.4.1. Control PES Membrane Preparation
3.4.2. PES hybrid Membranes Preparation
3.5. Characterization of Crude Cannabinoids and Terpenes
3.6. Characterization of Hybrid Membranes
3.6.1. FTIR Analysis
3.6.2. EDX Analysis
3.6.3. SEM
3.7. Mechanical Testing
3.8. Contact Angle Water Retention and Surface Roughness
3.9. X-ray Fluorescence (XRF) Test
3.10. Membrane Flux Test
3.11. Quantitative Bacterial Decline Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zameer, M.; Mahmood, S.; Mushtaq, Z.; Tabasum, B.; Ali, Q.; Mahmood, N.; Jamil, M.; Munir., S. Detection of bacterial load in drinking water samples of by 16sRNA ribotyping and RAPD analysis. Adv. Life Sci. 2015, 2, 135–141. [Google Scholar]
- Montgomery, M.A.; Elimelech, M. Water and Sanitation in Developing Countries: Including Health in the Equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Baldursson, S.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2004–2010. Water Res. 2011, 45, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Balkhair, K.S.; Albeiruttye, M.H.; Shaiban, A.A.J.; Shaiban, M.H.A.A.A.A.J. Importance and Significance of UF/MF Membrane Systems in Desalination Water Treatment. Desalination 2017. [Google Scholar] [CrossRef] [Green Version]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanoparticle Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Sun, P.-C.; Wei, J.-F. New insight into the fouling behavior of hydrophobic and hydrophilic polypropylene membranes in integrated membrane bioreactors. Environ. Technol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.T.; Ahmad, N.M.; Niazi, M.B.K.; Alvi, M.A.U.R.; Ahmad, N.; Anwar, M.N.; Cheema, W.; Tariq, S.; Batool, M.; Aman, Z.; et al. Graphene Oxide-PES-Based Mixed Matrix Membranes for Controllable Antibacterial Activity against Salmonella typhi and Water Treatment. Int. J. Polym. Sci. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Milescu, R.A.; McElroy, C.R.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP Water Filtration Membranes Using Cyrene®, a Safer Bio-Based Polar Aprotic Solvent. Adv. Polym. Technol. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Velu, S.; Muruganandam, L.; Arthanareeswaran, G. Preparation and Performance Studies on Polyethersulfone Ultrafiltration Membranes Modified with Gelatin for Treatment of Tannery and Distillery Wastewater. Braz. J. Chem. Eng. 2015, 32, 179–189. [Google Scholar] [CrossRef]
- Sabri, S.; Najjar, A.; Manawi, Y.; Eltai, N.O.; Al-Thani, A.; Atieh, M.A.; Kochkodan, V. Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum. Membranes 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.S.; Arthanareeswaran, G. Nano-curcumin incorporated polyethersulfone membranes for enhanced anti-biofouling in treatment of sewage plant effluent. Mater. Sci. Eng. C 2019, 94, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Franssen, M.C.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Boil. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Picot, M.; Rodulfo, R.; Nicolás, I.; Szymczyk, A.; Barriere, F.; Rabiller-Baudry, M. A versatile route to modify polyethersulfone membranes by chemical reduction of aryldiazonium salts. J. Membr. Sci. 2012, 417, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Van De Witte, P.; Dijkstra, P.; Berg, J.V.D.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Bilad, M.R.; Druyts, J.; Vankelecom, I.F.J. Role of Surface Pores on Fouling of Polyvinylidene Fluoride Membranes in Submerged Membrane Bioreactors. IOP Conf. Ser. Mater. Sci. Eng. 2017, 180, 12272. [Google Scholar] [CrossRef] [Green Version]
- Omidvar, M.; Soltanieh, M.; Mousavi, S.M.; Saljoughi, E.; Moarefian, A.; Saffaran, H. Preparation of hydrophilic nanofiltration membranes for removal of pharmaceuticals from water. J. Environ. Heal. Sci. Eng. 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudian, M.; Kochameshki, M.G.; Mahdavi, H.; Vahabi, H.; Enayati, M. Investigation of structure-performance properties of a special type of polysulfone blended membranes. Polym. Adv. Technol. 2018, 29, 2690–2700. [Google Scholar] [CrossRef]
- Cabasso, I.; Klein, E.; Smith, J.K. Polysulfone hollow fibers. I. Spinning and properties. J. Appl. Polym. Sci. 1976, 20, 2377–2394. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Membr. Sci. 2008, 320, 363–371. [Google Scholar] [CrossRef]
- Idarraga-Mora, J.A.; Childress, A.S.; Friedel, P.S.; Ladner, D.A.; Rao, A.M.; Husson, S.M. Role of Nanocomposite Support Stiffness on TFC Membrane Water Permeance. Membranes 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties in Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [Green Version]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Rahimi, M.; Dadari, S.; Zeinaddini, S.; Mohamadian, E. Flux, antifouling and separation characteristics enhancement of nanocomposite polyethersulfone mixed-matrix membrane by embedding synthesized hydrophilic adipate ferroxane nanoparticles. Korean J. Chem. Eng. 2017, 34, 1444–1455. [Google Scholar] [CrossRef]
- Purkait, M.; Sinha, M.; Mondal, P.; Singh, R. Introduction to Membranes. Interface Sci. Technol. 2018, 35, 1–37. [Google Scholar]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Preparation of a Novel Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane Modified with Reduced Graphene Oxide/Titanium Dioxide (TiO2) Nanocomposite with Enhanced Hydrophilicity and Antifouling Properties. Ind. Eng. Chem. Res. 2014, 53, 13370–13382. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes by Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Appendino, G.B.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Cunliffe, D.; Smart, C.A.; Alexander, C.; Vulfson, E.N. Bacterial Adhesion at Synthetic Surfaces. Appl. Environ. Microbiol. 1999, 65, 4995–5002. [Google Scholar] [CrossRef] [Green Version]
- Zerihun, A.; Chandravanshi, B.S.; Debebe, A.; Mehari, B. Levels of selected metals in leaves of Cannabis sativa L. cultivated in Ethiopia. SpringerPlus 2015, 4, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokate, C.; Purohit, A.; Gokhale, S. Pharmacognosy; Nirali Prakashan: Pune, India, 2008. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Abdala, A.; Hilal, N.; Khraisheh, M. Mechanical Characterization of Membranes. Membr. Charact. 2017, 2017, 259–306. [Google Scholar]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T.; et al. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef]
- Ovez, S.; Turken, T.; Kose-Mutlu, B.; Okatan, S.; Durmaz, G.; Guclu, M.C.; Guclu, S.; Chellam, S.; Koyuncu, I. Manufacturing of antibiofouling polymeric membranes with bismuth-BAL chelate (BisBAL). Desalin. Water Treat. 2015, 57, 1–15. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Sr. No. | Membrane Type | Average Width (µm) | Average Pore Size (µm) |
|---|---|---|---|
| 1 | PES | 34.27 | 1.03 |
| 2 | T | 39.45 | 0.97 |
| 3 | C | 50.35 | 0.63 |
| 4 | CT | 84.08 | 0.047 |
| Sr. No. | Membrane Type | Average Width (µm) | Average Pore Size (µm) |
|---|---|---|---|
| 1 | PES | 34.27 | 1.03 |
| 2 | T | 39.45 | 0.97 |
| 3 | C | 50.35 | 0.63 |
| 4 | CT | 84.08 | 0.047 |
| Sr. No. | Membrane | Average Contact Angle | Water Retention (%) | Surface Roughness Ra (nm) |
|---|---|---|---|---|
| 1 | PES | 93 | 55 | 36,880 |
| 2 | C-PES | 60 | 58 | 7650 |
| 3 | T-PES | 56 | 68 | 4332 |
| 4 | CT-PES | 40 | 70 | 2170 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes. Molecules 2020, 25, 691. https://doi.org/10.3390/molecules25030691
Nadir I, Rana NF, Ahmad NM, Tanweer T, Batool A, Taimoor Z, Riaz S, Ali SM. Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes. Molecules. 2020; 25(3):691. https://doi.org/10.3390/molecules25030691
Chicago/Turabian StyleNadir, Ismara, Nosheen Fatima Rana, Nasir Mahmood Ahmad, Tahreem Tanweer, Amna Batool, Zara Taimoor, Sundus Riaz, and Syed Mohsin Ali. 2020. "Cannabinoids and Terpenes as an Antibacterial and Antibiofouling Promotor for PES Water Filtration Membranes" Molecules 25, no. 3: 691. https://doi.org/10.3390/molecules25030691