Syntheses, Structures, and Characteristics of Three Metal Complexes Constructed Using Hexacarboxylic Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of {[Cd3(L)(H2O)6]·H2O}n (1)
2.3. Synthesis of {[Cu1.5(L)0.5(bimb)1.5]·5H2O·DMF}n (2)
2.4. Synthesis of {[Mn1.5(H3L)(bibp)0.5(H2O)2]·3H2O}n (3)
2.5. X-ray Crystallography
3. Results and Discussion
3.1. Structural Description of 1
3.2. Structural Description of 2
3.3. Structural Description of 3
3.4. PXRD and Thermogravimetric Analysis (TGA)
3.5. Photoluminescent Properties of 1
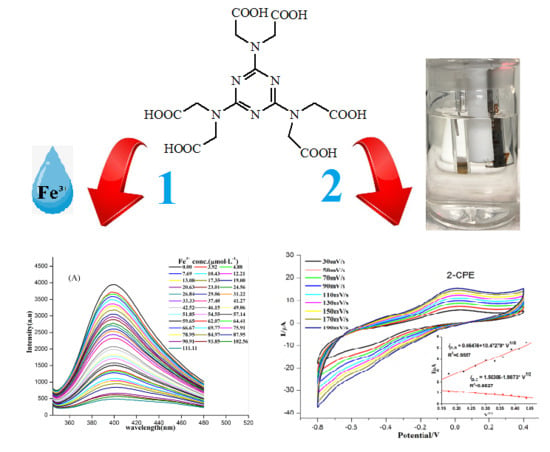
3.6. Detection of Fe3+ Ions
3.7. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goswami, P.K.; Singh, M.; Thaimattam, R.; Ramanan, A. Extending the supramolecular synthon concept in flexible polyaminocarboxylate based coordination polymers. CrystEngComm 2013, 15, 9787–9797. [Google Scholar] [CrossRef]
- Han, S.D.; Song, W.C.; Zhao, J.P.; Yang, Q.; Liu, S.J.; Lia, Y.; Bu, X.H. Synthesis and ferrimagnetic properties of an unprecedented polynuclear cobalt complex composed of [Co24] macrocycles. Chem. Commun. 2013, 49, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Gole, B.; Sanyal, U.; Banerjee, R.; Mukherjee, P.S. High Loading of Pd Nanoparticles by Interior Functionalization of MOFs for Heterogeneous Catalysis. Inorg. Chem. 2016, 55, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Zamora, F. Coordination polymers with nucleobases: From structural aspects to potential applications. Coord. Chem. Rev. 2014, 276, 34–58. [Google Scholar] [CrossRef]
- Zhou, H.C.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, G. Double-Stranded Helices and Molecular Zippers Assembled from Single-Stranded Coordination Polymers Directed by Supramolecular Interactions. Chem. Eur. J. 2002, 8, 4811–4817. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A. Modulating methane storage in anionic nano-porous MOF materials via post-synthetic cation exchange process. Dalton Trans. 2013, 42, 4786–4789. [Google Scholar] [CrossRef]
- Keskin, S.; Sholl, D.S. Selecting metal organic frameworks as enabling materials in mixed matrix membranes for high efficiency natural gas purification. Energy Environ. Sci. 2010, 3, 343–351. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.R.; Li, J.R.; Makal, T.A.; Young, M.D.; Yuan, D.Q.; Zhao, D.; Zhuang, W.J.; Zhou, H.C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Jiang, J.; Yaghi, O.M. Bronsted Acidity in Metal-Organic Frameworks. Cheminform 2015, 115, 6966–6997. [Google Scholar] [CrossRef]
- Hill, R.J.; Long, D.L.; Champness, N.R.; Hubberstey, P.R. New Approaches to the Analysis of High Connectivity Materials: Design Frameworks Based upon 44- and 63-Subnet Tectons. Chem. Rev. 2005, 38, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wei, Y.L.; Dong, X.Y.; Zang, S.Q.; Mak, T.C.W. Novel Tb-MOF Embedded with Viologen Species for Multi-Photofunctionality: Photochromism, Photomodulated Fluorescence, and Luminescent pH Sensing. Chem. Mater. 2015, 27, 1327–1331. [Google Scholar] [CrossRef]
- Rhauderwiek, T.; Heidenreich, N.; Reinsch, H.; Øien-Ødegaard, S.; Lomachenko, K.A.; Rütt, U.; Soldatov, A.V.; Lillerud, K.P.; Stock, N. Co-ligand dependent Formation and Phase Transformation of Four Porphyrin-based Cerium MOFs. Cryst. Growth Des. 2017, 17, 3462–3474. [Google Scholar] [CrossRef]
- Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Topological Analysis of Metal–Organic Frameworks with Polytopic Linkers and/or Multiple Building Units and the Minimal Transitivity Principle. Chem. Rev. 2014, 114, 1343–1370. [Google Scholar] [CrossRef]
- Wang, X.S.; Chrzanowski, M.; Kim, C.; Gao, W.Y.; Wojtas, L.; Chen, Y.S.; Zhanga, X.P.; Ma, S. Quest for highly porous metal–metalloporphyrin framework based upon a custom-designed octatopic porphyrin ligand. Chem. Commun. 2012, 48, 7173–7175. [Google Scholar] [CrossRef]
- Chen, Z.; Mi, X.; Lu, J.; Wang, S.; Li, Y.; Doua, J.; Lia, D. From 2D→ 3D interpenetration to packing: N coligand-driven structural assembly and tuning of luminescent sensing activities towards Fe3+ and Cr2O72− ions. Dalton Trans. 2018, 47, 6240–6249. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.Q.; Guo, N.; Sun, Y.L.; Zhang, T.; Ma, L.F.; Wang, L.Y. Series d–f Heteronuclear Metal–Organic Frameworks: Color Tunability and Luminescent Probe with Switchable Properties. Inorg. Chem. 2017, 56, 1713–1721. [Google Scholar] [CrossRef]
- Zheng, T.T.; Zhao, J.; Fang, Z.W.; Li, M.T.; Sun, C.Y.; Li, X.; Wang, X.L.; Su, Z.M. A luminescent metal organic framework with high sensitivity for detecting and removing copper ions from simulated biological fluids. Dalton Trans. 2017, 46, 2456–2461. [Google Scholar] [CrossRef]
- Chen, S.G.; Shi, Z.Z.; Qin, L.; Jia, H.L.; Zheng, H.G. Two New Luminescent Cd(II)-MOFs as Bi-functional Chemosensors for Detection of Cations Fe3+, Anions CrO42− and Cr2O72− in Aqueous Solution. Cryst. Growth. Des. 2017, 17, 67. [Google Scholar] [CrossRef]
- Hyman, L.; Franz, K. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [Green Version]
- Yi, F.Y.; Yang, W.; Sun, Z.M. Highly selective acetone fluorescent sensors based on microporous Cd(ii) metal–organic frameworks. J. Mater. Chem. 2012, 22, 23201–23209. [Google Scholar] [CrossRef]
- Hua, J.A.; Zhao, Y.; Kang, Y.S.; Lu, Y.; Sun, W.Y. Solvent-dependent zinc(II) coordination polymers with mixed ligands: Selective sorption and fluorescence sensing. Dalton Trans. 2015, 44, 11524–11532. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.X.; Wu, Y.P.; Dong, W.W.; Zhao, J.; Li, D.S.; Zhang, J. An Ultrastable Europium(III)–Organic Framework with the Capacity of Discriminating Fe2+/Fe3+ Ions in Various Solutions. Inorg. Chem. 2016, 55, 10114–10117. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bon, A.; Costero, A.M.; Gil, S. A new selective fluorogenic probe for trivalent cations. Chem. Commun. 2012, 48, 3000–3002. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.L.; Shi, Y.X.; Chen, H.H.; Lang, J.P. A Zn(II) coordination polymer and its photocycloaddition product: Syntheses, structures, selective luminescence sensing of iron(III) ions and selective absorption of dyes. Dalton Trans. 2015, 44, 18795–18803. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tian, W.G.; Liu, X.X.; Wang, L.; Sun, Z.M. Syntheses, Structures, Luminescence, and Photocatalytic Properties of a Series of Uranyl Coordination Polymers. Cryst. Growth Des. 2014, 14, 5904–5911. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Yan, L.; Peng, R.; Huangfu, M.J.; Guo, X.X.; Li, Y.; Wu, P.Y. Multifunctional Luminescent Eu(III)-Based Metal–Organic Framework for Sensing Methanol and Detection and Adsorption of Fe(III) Ions in Aqueous Solution. Inorg. Chem. 2016, 55, 12660–12668. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.R.; Li, Y.; Jiang, M.; Zhang, L.W.; Wu, P.Y. A europium(III)-based metal–organic framework as a naked-eye and fast response luminescence sensor for acetone and ferric iron. New J. Chem. 2016, 40, 8600–8606. [Google Scholar] [CrossRef]
- Hao, Z.M.; Yang, G.C.; Song, X.Z.; Zhu, M.; Meng, X.; Zhao, S.N. A europium(III) based metal–organic framework: Bifunctional properties related to sensing and electronic conductivity. J. Mater. Chem. A 2014, 2, 237–244. [Google Scholar] [CrossRef]
- Liang, Y.T.; Yang, G.P.; Liu, B.; Yan, Y.T.; Xi, Z.P.; Wang, Y.Y. Four super water-stable lanthanide–organic frameworks with active uncoordinated carboxylic and pyridyl groups for selective luminescence sensing of Fe3+. Dalton Trans. 2015, 44, 13325–13330. [Google Scholar] [CrossRef]
- Zhang, J.N.; Lu, H.B.; Yan, C.; Yang, Z.B.; Zhu, G.Q.; Gao, J.Z.; Yin, F.; Wang, C.L. Fabrication of conductive graphene oxide-WO3 composite nanofibers by electrospinning and their enhanced acetone gas sensing properties. Sens. Actuators B. 2018, 264, 128–138. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Büschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the Development of Sensor Molecules that Display Fe(III)-amplified Fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, J.; Ni, C.; Wang, Z.N.; Gao, X.; Shi, Z.; Bai, F.Y.; Xing, Y.H. Photoelectric properties and potential nitro derivatives sensing by a highly luminescent of Zn (II) and Cd (II) metal-organic frameworks assembled by the flexible hexapodal ligand, 1,3,5-triazine-2,4,6-triamine hexaacetic acid. RSC Adv. 2016, 6, 36000–36010. [Google Scholar] [CrossRef]
- Song, J.; Gao, X.; Wang, Z.N.; Li, C.R.; Xu, Q.; Bai, F.Y.; Shi, Z.F.; Xing, Y.H. Multifunctional Uranyl Hybrid Materials: Struc tural Diversities as a Function of pH, Luminescence with Potential Nitrobenzene Sensing, and Photoelectric Behavior as p-type Semiconductors. Inorg. Chem. 2015, 54, 9046–9059. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, N.; Tse, K.M.; Lee, H.P. Hydrothermal synthesis, crystal structures, and luminescent properties of two cadmium(ii) coordination polymers based on dicarboxylate and imidazole-containing coligands. Z. Anorg. Allg. Chem. 2015, 641, 601–605. [Google Scholar] [CrossRef]
- Li, X.C.; Zheng, J.; He, C.J.; Wang, K.; Chai, W.W.; Duan, Y.T.; Tang, B.J.; Rui, Y.C. MOF-derived Cu–C loaded with SnOx as a superior anode material for lithium-ion batteries. Electrochim. Acta 2019, 326, 134960. [Google Scholar] [CrossRef]
- Rambabu, D.; Ashraf, M.; Pooja, G.A.; Dhir, A. Mn-MOF@Pi composite: Synthesis, characterisation and an efficient catalyst for the Knoevenagel condensation reaction. Tetrahedron Lett. 2017, 58, 4691–4694. [Google Scholar] [CrossRef]
- Meng, L.S.; Zhao, L.; Zhao, C.J.; Lin, X. Synthesis, characterization and electrochemical properties of two metal cobalt complexes constructed by tetradentate carboxylic. J. Mol. Struct. 2019, 1179, 425–430. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.P.; Yang, Z.M.; Yao, P.F.; Yu, Q.; Tian, J.L.; Bian, H.D.; Yan, S.P.; Liao, D.Z.; Cheng, P. Coordination assemblies of the CdII–BDC/bpt mixed-ligand system: Positional isomeric effect, structural diversification and luminescent properties. CrystEngComm 2013, 15, 2657–2688. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Li, Y.; Zhu, A.Z.; Meng, Q. Four d10 Metal Coordination Polymers Containing IsomericThiodiphthalic Ligands: Crystal Structures and LuminescentProperties. Cryst. Growth Des. 2007, 7, 1277–1283. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.T.; Yang, J.; Wu, H.; Liu, Y.Y.; Ma, J.F. Systematic investigation of high-sensitivity luminescent sensing for polyoxometalates and Iron(III) by MOFs assembled with a new resorcin[4]arene-functionalized tetracarboxylate. Chem. Eur. J. 2015, 21, 15806–15819. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Shi, W.; Li, H.M.; Li, H. Experimental studies and mechanism analysis of high-sensitivity luminescent sensing of pollutional small molecules and ions in Ln4O4 cluster based microporous metal-organic framework. J. Phys. Chem. C 2014, 118, 416–426. [Google Scholar] [CrossRef]
- Zhao, X.L.; Tian, D.; Gao, Q.; Sun, H.W. A chiral lanthanide metal-organic framework for selective sensing of Fe(III) ion. Dalton Trans. 2016, 45, 1040–1046. [Google Scholar] [CrossRef]
- Das, D.; Biradha, K. Luminescent Coordination polymers of naphthalene based diamide with rigid and flexible dicarboxylates: Sensing of nitro explosives, Fe(III) Ion, and dyes. Cryst. Growth Des. 2018, 18, 3683–3692. [Google Scholar] [CrossRef]
- Salimi, A.; Alizadeh, V.; Hadadzadeh, H. Renewable Surface Sol-gel Derived Carbon Ceramic Electrode Modified with Copper Complex and Its Application as an Amperometric Sensor for Bromate Detection. Electroanalysis 2004, 16, 1984–1991. [Google Scholar] [CrossRef]
- Wang, X.L.; Mu, B.; Lin, H.Y.; Liu, G.C. Three new two-dimensional metal-organic coordination polymers derived from bis(pyridinecarboxamide)-1,4-benzene ligands and 1,3-benzenedicarboxylate: Syntheses and electrochemical property. J. Organomet. Chem. 2011, 696, 2313–2321. [Google Scholar] [CrossRef]








| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Molecular Formula | C15H26Cd3N6O19 | C57H64N20Cu3O24 | C54H50Mn3N18O34 |
| Formula weight | 917.54 | 1593.85 | 1659.94 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/c | C2/c | P-1 |
| a/Å | 9.7574 (3) | 31.421 (3) | 8.234 (2) |
| b/Å | 15.1249 (5) | 17.6474 (18) | 10.610 (3) |
| c/Å | 18.6267 (6) | 17.2392 (17) | 19.504 (6) |
| α/(°) | 90 | 90 | 81.094 (6) |
| β/(°) | 95.449 (1) | 117.491 (2) | 87.935 (7) |
| γ/(°) | 90 | 90 | 86.408 (7) |
| V/nm3 | 2736.50 (15) | 8479.7 (15) | 1679.6 (8) |
| Z | 4 | 4 | 1 |
| Dc/(g·cm−3) | 2.227 | 1.248 | 1.641 |
| F(000) | 1760 | 3260 | 847 |
| s | 1.090 | 1.189 | 1.039 |
| R1/wR2 [I > 2σ(I)] | 0.0235, 0.0623 | 0.1020, 0.3087 | 0.0682, 0.1824 |
| R1/wR2 (all data) | 0.0268, 0.0640 | 0.1684, 0.3611 | 0.1115, 0.2223 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Zhao, L.; Guo, G.; Liu, X.; Liang, Z.; Xiu, J.; Zhou, X. Syntheses, Structures, and Characteristics of Three Metal Complexes Constructed Using Hexacarboxylic Acid. Molecules 2019, 24, 4431. https://doi.org/10.3390/molecules24244431
Meng L, Zhao L, Guo G, Liu X, Liang Z, Xiu J, Zhou X. Syntheses, Structures, and Characteristics of Three Metal Complexes Constructed Using Hexacarboxylic Acid. Molecules. 2019; 24(24):4431. https://doi.org/10.3390/molecules24244431
Chicago/Turabian StyleMeng, Lingshu, Lun Zhao, Guanlin Guo, Xin Liu, Zhijun Liang, Jian Xiu, and Xu Zhou. 2019. "Syntheses, Structures, and Characteristics of Three Metal Complexes Constructed Using Hexacarboxylic Acid" Molecules 24, no. 24: 4431. https://doi.org/10.3390/molecules24244431