Effects of Orally Consumed Rosa damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
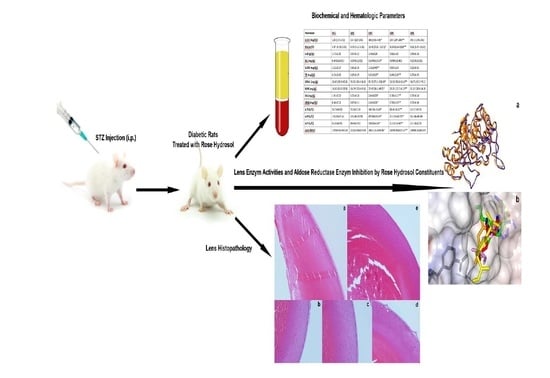
2. Results
2.1. Quantifying Rose Hydrosol Volatiles
2.2. Water Intakes and Body Weights
2.3. Hematological Parameters
2.4. Biochemical Parameters
2.5. Lens enzymatic Activities
2.6. Lens Pathology
- Grade 0: Presence of anterior epithelium with lens fibers and capsule;
- Grade 1: Presence of anterior epithelium, lens fibers, vacuoles, and capsule;
- Grade 2: Presence of anterior epithelium, lens fibers, vacuoles, homogenized areas, and capsule;
- Grade 3: Absence of anterior epithelium, presence of lens fibers, vacuoles, homogenized area;
- Grade 4: Presence of lens fibers and homogenized area only.
2.7. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Animals and Induction and Diabetes
4.2. Experimental Protocol
4.3. Blood Sampling Procedures for Hematology, Clinical Biochemistry, and AGE Formation
4.4. Collecting the Lenses for Enzymatic Activity and Eyes for Lens Pathology
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Pollreisz, A.; Schmidt-Erfurth, U. Diabetic cataract—pathogenesis, epidemiology and treatment. J. Ophthalmol. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Dean, R. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Mullarkey, C.J.; Edelstein, D.; Brownlee, M. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem. Biophys. Res. Commun. 1990, 173, 932–939. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Lyons, T.J. Glycation, oxidation, and lipoxidation in the development of diabetic complications. Metabolism 1997, 46, 14–21. [Google Scholar] [CrossRef]
- Kawamura, N.; Ookawara, T.; Suzuki, K.; Konishi, K.; Mino, M.; Taniguchi, N. Increased glycated Cu, Zn-superoxide dismutase levels in erythrocytes of patients with insulin-dependent diabetis mellitus. J. Clin. Endocrinol. Metab. 1992, 74, 1352–1354. [Google Scholar]
- Morgan, P.E.; Dean, R.T.; Davies, M.J. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch. Biochem. Biophys. 2002, 403, 259–269. [Google Scholar] [CrossRef]
- Kinoshita, J.H. Cataracts in galactosemia: The Jonas, S. Friedenwald Meml. Lecture. Investig. Ophthalmol. Vis. Sci. 1965, 4, 786–799. [Google Scholar]
- Kinoshita, J.H. Mechanisms initiating cataract formation proctor lecture. Investig. Ophthalmol. Vis. Sci. 1974, 13, 713–724. [Google Scholar]
- Kinoshita, J.H.; Fukushi, S.; Kador, P.; Merola, L.O. Aldose reductase in diabetic complications of the eye. Metabolism 1979, 28, 462–469. [Google Scholar] [CrossRef]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14, S233–S236. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Altintas, A.; Kurkcuoglu, M. Turkish rose: A review of the history, ethnobotany and modern uses of rose petals, rose oil, rose water and other rose products. HerbalGram 2012, 96, 40–53. [Google Scholar]
- Altintas, A. Rose, Rose Water, Historical, Therapeutic and Cultural Perspectives; Maestro Publishing: Istanbul, Turkey, 2010. [Google Scholar]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295. [Google Scholar]
- Gholamhoseinian, A.; Fallah, H. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine 2009, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.G.; Gupta, A.; Kapahi, B.K.; Baleshwar; Thappa, R.K.; Suri, O.P. Chemical Composition of Rose Water Volatiles. J. Essent. Oil Res. 2005, 17, 265–267. [Google Scholar] [CrossRef]
- Habtemariam, S. Antidiabetic potential of monoterpenes: A case of small molecules punching above their weight. Int. J. Mol. Sci. 2017, 19, 4. [Google Scholar] [CrossRef]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, S.; Muruganathan, U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2016, 250, 38–46. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sathish, G.; Jayanthi, M.; Muthukumaran, J.; Muruganathan, U.; Ramachandran, V. Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2014, 385, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Canbek, M.; Uyanoglu, M.; Canbek, S.; Ceyhan, E.; Ozen, A.; Durmus, B.; Turgak, O. The Effect of Geraniol on Liver Regeneration after Hepatectomy in Rats. In vivo (Athens, Greece) 2017, 31, 209–213. [Google Scholar]
- Senol, F.S.; Orhan, I.E.; Kurkcuoglu, M.; Khan, M.T.H.; Altintas, A.; Sener, B.; Baser, K.H.C. A mechanistic investigation on anticholinesterase and antioxidant effects of rose (Rosa damascena Mill.). Food Res. Int. 2013, 53, 502–509. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.V. Haematological profile of diabetes and non-diabetes patients in rural tertiary centre. Int. J. Adv. Med. 2017, 4, 1271. [Google Scholar] [Green Version]
- Milosevic, D.; Panin, V.L. Relationship between hematological parameters and glycemic control in type 2 diabetes mellitus patients. J. Med. Biochem. 2019, 38, 164. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, L.; Degirmenci, H.; Akbas, E.M.; Ozcicek, A.; Timuroglu, A.; Gurel, A.; Ozcicek, F. Association of hematological indicies with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int. J. Clin. Exp. Med. 2015, 8, 11420. [Google Scholar]
- Bernardi, C.; Monetal, D.; Brughera, M.; Di Salvo, M.; Lamparelli, D.; Mazue, G.; Iatropoulos, M. Haematology and clinical chemistry in rats: Comparison of different blood collection sites. Comp. Haematol. Int. 1996, 6, 160–166. [Google Scholar] [CrossRef]
- Jacob Filho, W.; Lima, C.C.; Paunksnis, M.R.R.; Silva, A.A.; Perilhão, M.S.; Caldeira, M.; Bocalini, D.; de Souza, R.R. Reference database of hematological parameters for growing and aging rats. Aging Male 2018, 21, 145–148. [Google Scholar] [CrossRef]
- Lillie, L.E.; Temple, N.J.; Florence, L.Z. Reference values for young normal Sprague-Dawley rats: Weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1996, 15, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Keskin, E.; Dönmez, N.G.; Kandır, S. Beneficial Effect of Quercetin on Some Haematological Parameters in Streptozotocin-Induced Diabetic Rats. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 65–68. [Google Scholar]
- Mahmoud, A.M. Hematological alterations in diabetic rats-role of adipocytokines and effect of citrus flavonoids. EXCLI J. 2013, 12, 647. [Google Scholar] [PubMed]
- World Health Organization. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: Abbreviated report of a WHO consultation; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Saudek, C.D.; Derr, R.L.; Kalyani, R.R. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. Jama 2006, 295, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.H.; Budin, S.B. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132. [Google Scholar] [CrossRef]
- Harris, E.H. Elevated liver function tests in type 2 diabetes. Clin. Diabetes 2005, 23, 115–119. [Google Scholar] [CrossRef]
- Dabla, P.K. Renal function in diabetic nephropathy. World J. Diabetes 2010, 1, 48. [Google Scholar] [CrossRef]
- Katsiki, N.; Papanas, N.; Fonseca, V.A.; Maltezos, E.; Mikhailidis, D.P. Uric acid and diabetes: Is there a link? Curr. Pharm. Des. 2013, 19, 4930–4937. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Derm. Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Neves, D. Advanced glycation end-products: A common pathway in diabetes and age-related erectile dysfunction. Free Radic. Res. 2013, 47, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Höhn, A. Role of Advanced Glycation End Products in Carcinogenesis and their Therapeutic Implications. Curr. Pharm. Des. 2018, 24, 5245–5251. [Google Scholar] [CrossRef] [PubMed]
- Morimitsu, Y.; Yoshida, K.; Esaki, S.; Hirota, A. Protein glycation inhibitors from thyme (Thymus vulgaris). Biosci. Biotechnol. Biochem. 1995, 59, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Devamanoharan, P.S.; Henein, M.; Ali, A.H.; Varma, S.D. Diabetes-induced biochemical changes in rat lens: Attenuation of cataractogenesis by pyruvate. Diabetes Obes. Metab. 2000, 2, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts: BI 2015, 5, 123. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88. [Google Scholar]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Jurkovič, S.; Osredkar, J.; Marc, J. Molecular impact of glutathione peroxidases in antioxidant processes. Biochem. Med.: Biochem. Med. 2008, 18, 162–174. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813. [Google Scholar] [CrossRef] [PubMed]
- Kalousova, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Agarwal, R.; Iezhitsa, I.; Awaludin, N.A.; Fisol, N.F.A.; Bakar, N.S.; Agarwal, P.; Rahman, T.H.A.; Spasov, A.; Ozerov, A.; Salama, M.S.M.A. Effects of magnesium taurate on the onset and progression of galactose-induced experimental cataract: In vivo and in vitro evaluation. Exp. Eye Res. 2013, 110, 35–43. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Group | Body Weight (Start–End) (g ± SD) | Average Water Intake (ml ± SD) | Daily Average Rose Hydrosol Volatiles Intake (mg ± SD) |
|---|---|---|---|
| GR1 (Normal rats supplied with tap water) | 236 ± 13–367 ± 34 | 42 ± 5 | None |
| GR2 (Normal rats supplied with RH1) | 236 ± 13–359 ± 42 | 38 ± 3 | 57.5 ± 5 |
| GR3 (Diabetic rats supplied tap with water) | 236 ± 13–178 ± 27 * | 125 ± 12 * | None |
| GR4 (Diabetic rats supplied with RH1) | 236 ± 13–227 ± 12 ** | 73 ± 23 ** | 110 ± 35 |
| GR5 (Diabetic rats supplied with RH2) | 236 ± 13–208 ± 25 | 81 ± 12 | 40.5 ± 6 |
| Parameter | GR1 | GR2 | GR3 | GR4 | GR5 |
|---|---|---|---|---|---|
| WBC (103/µL) | 6.26 ± 1.31 | 7.07 ± 1.23 | 9.72 ± 1.27 * | 7.93 ± 0.59 ** | 7.99 ± 1.61 |
| RBC (106/µL) | 8.11 ± 0.39 | 8.60 ± 0.46 | 6.19 ± 0.75 * | 8.39 ± 0.42 ** | 8.54 ± 0.52 |
| HGB (g/dL) | 14.43 ± 0.52 | 14.79 ± 0.67 | 9.44 ± 0.37 * | 14.17 ± 1.37 ** | 14.86 ± 0.96 |
| HCT (%) | 45.84 ± 1.37 | 46.57 ± 1.92 | 46.86 ± 2.87 | 45.73 ± 1.45 | 46.80 ± 2.24 |
| MCV (fl) | 56.58 ± 1.94 | 56.15 ± 1.98 | 56.05 ± 1.45 | 56.00 ± 1.48 | 55.43 ± 1.16 |
| MCH (pg) | 17.80 ± 0.38 | 17.67 ± 0.52 | 17.78 ± 0.46 | 17.68 ± 0.37 | 17.72 ± 0.35 |
| MCHC (g/dL) | 31.55 (30.6–32.3) | 31.80 (31.2–32.3) | 31.85 (30.2–32.7) | 31.80 (30.2–32.1) | 32.30 (30.1–33.5) |
| PLT (103/µL) | 760.10 ± 94.74 | 772.30 ± 88.97 | 339.70 ± 100.06 * | 735.10 ± 92.98 ** | 670.70 ± 181.14 |
| RDW-SD (fl) | 31.31 ± 1.38 | 30.30 ± 0.92 | 36.53 ± 2.69 * | 30.93 ± 1.28 ** | 32.43 ± 1.42 |
| RDW-CV (%) | 17.99 ± 0.69 | 18.44 ± 0.69 | 20.44 ± 1.69 * | 18.22 ± 1.03 ** | 19.37 ± 0.94 |
| PDW (fl) | 10.30 (9.4–11.6) | 10.86 (10.6–12.7) | 11.75 (10.4–21.1) | 10.65 (9.4–11.4) | 11.45 (9.0–13.4) |
| MPV (fl) | 9.15 (8.6–10.0) | 9.60 (9.3–10.6) | 9.55 (9.2–10.1) | 9.35 (8.6–9.9) | 9.55 (9.0–10.2) |
| P-LCR (%) | 18.80 (14.7–25.7) | 22.35 (20.2–30.8) | 24.05 (18.9–26.9) * | 20.40 (14.7–24.6) | 22.20 (17.8–28.9) |
| PCT (%) | 0.71 (0.60–0.94) | 0.74 (0.60–0.94) | 0.77 (0.59–0.83) | 0.70 (0.60–0.86) | 0.48 (0.30–0.90) |
| NEUT (%) | 41.97 ± 2.78 | 42.07 ± 3.05 | 49.52 ± 1.82 | 42.78 ± 2.41 | 40.72 ± 2.40 |
| LYMPH (%) | 48.18 (46.09–49.86) | 47.94 (46.19–49.94) | 41.35 (38.53–43.86) | 47.30 (46.43–49.92) | 47.92 (46.09–48.99) |
| MONO (%) | 8.73 ± 2.34 | 9.01 ± 2.61 | 8.67 ± 1.09 | 8.61 ± 2.20 | 10.19 ± 2.51 |
| EO (%) | 0.90 (0.2–6.40) | 1.10 (0.2–1.80) | 0.30 (0.10–0.60) | 1.30 (0.40–2.20) | 0.80 (0.06–6.10) |
| BASO (%) | 0.00 (0.0–1.0) | 0.00 (0.0–0.0) | 0.00 (0.0–1.0) | 0.00 (0.0–0.0) | 0.00 (0.0–1.0) |
| Parameter | GR1 | GR2 | GR3 | GR4 | GR5 |
|---|---|---|---|---|---|
| GLUC (mg/dL) | 129 (115–141) | 134 (122–146) | 383 (361–434) * | 259 (227–289) ** | 292.5 (276–351) |
| HbA1c (%) | 4.97 (4.11–5.83) | 4.73 (4.15–5.31) | 11.41 (9.31–13.51) * | 8.34 (8.56–9.90) ** | 9.61 (8.97–10.02) |
| ALB (g/dL) | 3.72 ± 0.25 | 3.57 ± 0.22 | 3.48 ± 0.28 | 3.56 ± 0.42 | 3.49 ± 0.26 |
| BIL (mg/dL) | 0.0460 ± 0.013 | 0.0440 ± 0.012 | 0.0540 ± 0.016 * | 0.0480 ± 0.001 | 0.0520 ± 0.013 |
| GLOB (mg/dL) | 2.52 ± 0.37 | 2.66 ± 0.35 | 3.36 ± 0.40 ** | 2.87 ± 0.58 | 3.20 ± 0.61 |
| TP (mg/dL) | 6.24 ± 0.41 | 6.23 ± 0.29 | 6.85 ± 0.27 * | 6.44 ± 0.39 ** | 6.70 ± 0.45 |
| UREA (mg/dL) | 36.00 (28.3–40.6) | 35.95 (26.4–38.8) | 80.10 (73.1–106.0) * | 56.10 (50.8–66.6) ** | 68.75 (61.3–79.1) |
| BUN (mg/dL) | 16.82 (13.2–18.9) | 16.79 (12.8–19.0) | 37.43 (34.1–49.5) * | 26.21 (23.7–31.2) ** | 32.12 (28.6–36.9) |
| UA (mg/dL) | 1.01 ± 0.12 | 1.05 ± 0.18 | 2.04 ± 0.23 * | 1.36 ± 0.11 ** | 1.53 ± 0.18 |
| CREA (mg/dL) | 0.64 ± 0.12 | 0.67 ± 0.11 | 2.40 ± 0.21 * | 1.50 ± 0.26 ** | 2.03 ± 0.40 |
| ALT (U/L) | 54.74 ± 8.00 | 53.36 ± 7.38 | 141.13 ± 16.48 * | 86.41 ± 8.62 ** | 115.17 ± 9.71 |
| ALP (U/L) | 131.60 ± 25.41 | 119.80 ± 15.58 | 457.80 ± 99.89 * | 212.20 ± 40.75 ** | 333.00 ± 42.49 |
| AST (U/L) | 81.83 ± 6.55 | 80.48 ± 5.92 | 130.25 ± 8.60* | 113.67 ± 3.64 ** | 121.58 ± 2.79 |
| AGEs (RFU) | 13,739.33 ± 434.24 | 13,201.93 ± 325.08 | 24,315.31 ± 346.86 * | 18,950.85 ± 625.24 ** | 19,868.20 ± 683.05 |
| Parameter | GR1 | GR2 | GR3 | GR4 | GR5 |
|---|---|---|---|---|---|
| Lens Weight (mg) | 31 (30–33) | 30 (29–32) | 25 (23–28) * | 30 (29–32) ** | 31 (30–32) |
| Total Lens Protein (mg/100 mg lens) | 35.70 (33.10–37.52) | 34.55 (33.80–36.85) | 28.71 (27.08–30.57) * | 35.15 (33.05–37.66) ** | 36.38 (33.71–37.82) |
| MDA (µmole/g protein) | 2.89 (2.77–2.98) | 2.94 (2.80–3.06) | 3.01 (2.72–3.09) | 2.86 (2.74–2.94) | 2.97 (2.78–3.08) |
| GPx (mU/mg protein) | 4.16 ± 0.22 | 4.59 ± 0.33 | 2.11 ± 0.19* | 4.13 ± 0.64 ** | 3.67 ± 0.36 |
| SOD (U/mg protein) | 37.82 ± 3.16 | 37.75 ± 3.84 | 38.89 ± 3.34 | 39.36 ± 3.53 | 38.12 ± 2.78 |
| CAT (mU/mg protein) | 0.013 (0.009–0.015) | 0.019 (0.013–0.021)* | 0.010 (0.008–0.12) | 0.012 (0.009–0.014) | 0.011 (0.008–0.012) |
| AR (ng/mL) | 0.61 ± 0.05 | 0.49 ± 0.04 *** | 1.33 ± 0.32 * | 0.62 ± 0.04 ** | 0.68 ± 0.073 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirbolat, İ.; Ekinci, C.; Nuhoğlu, F.; Kartal, M.; Yıldız, P.; Geçer, M.Ö. Effects of Orally Consumed Rosa damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 4069. https://doi.org/10.3390/molecules24224069
Demirbolat İ, Ekinci C, Nuhoğlu F, Kartal M, Yıldız P, Geçer MÖ. Effects of Orally Consumed Rosa damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats. Molecules. 2019; 24(22):4069. https://doi.org/10.3390/molecules24224069
Chicago/Turabian StyleDemirbolat, İlker, Cansu Ekinci, Fadime Nuhoğlu, Murat Kartal, Pelin Yıldız, and Melin Özgün Geçer. 2019. "Effects of Orally Consumed Rosa damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats" Molecules 24, no. 22: 4069. https://doi.org/10.3390/molecules24224069