Development and Validation of a Reporter-Cell-Line-Based Bioassay for Therapeutic Soluble gp130-Fc
Abstract
:1. Introduction
2. Results
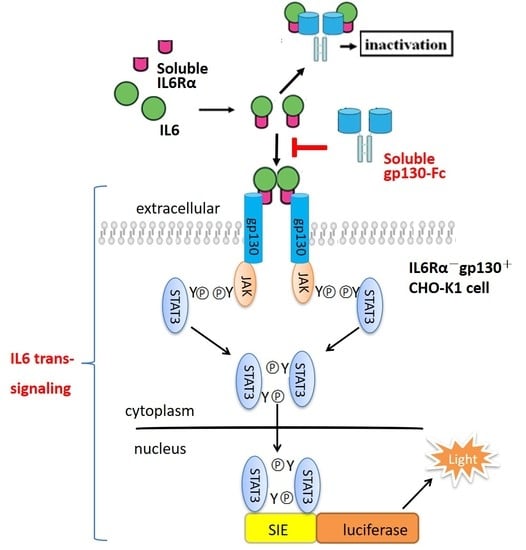
2.1. Development of a Stable Reporter Cell Line for sgp130-Fc
2.2. Method Development and Optimization
2.3. Method Validation
2.3.1. Specificity
2.3.2. Precision, Accuracy, and Linearity
2.3.3. Consistency with BAF3/gp130 Proliferation Assay in Test Results
2.4. Stability of CHO/SIE-Luc Cell Line
2.4.1. Function Stability
2.4.2. Genetic Stability
3. Discussion
4. Materials and Methods
4.1. Cells and Materials
4.2. Preparation of a Stable Reporter Cell Line
4.3. Reporter Assay
4.4. BAF3/gp130 Proliferation Assay
4.5. PCR Primers and Probes
4.6. Digital PCR
4.7. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahir-Jouzdani, F.; Atyabi, F.; Mojtabavi, N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology 2017, 24, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Puchner, A.; Bluml, S. IL-6 blockade in chronic inflammatory diseases. Wien. Med. Wochenschr. 2015, 165, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Jovani, M.; Fiorino, G.; Schreiber, S.; Danese, S. Anti-IL-6 treatment for inflammatory bowel diseases: Next cytokine, next target. Curr. Drug Targets 2013, 14, 1508–1521. [Google Scholar] [CrossRef]
- Kanda, T.; Takahashi, T. Interleukin-6 and cardiovascular diseases. Jpn. Heart J. 2004, 45, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Sun, Y.M. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review). Int. J. Oncol. 2014, 44, 1032–1040. [Google Scholar] [CrossRef]
- Liu, H.; Shen, J.; Lu, K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 2017, 486, 239–244. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Kampan, N.C.; Xiang, S.D.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Immunotherapeutic Interleukin-6 or Interleukin-6 Receptor Blockade in Cancer: Challenges and Opportunities. Curr. Med. Chem. 2018, 25, 4785–4806. [Google Scholar] [CrossRef]
- Taher, M.Y.; Davies, D.M.; Maher, J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem. Soc. Trans. 2018, 46, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor alpha to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.J.; Chow, D.C.; Brevnova, E.E.; Garcia, K.C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef]
- Scheller, J.; Ohnesorge, N.; Rose-John, S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand. J. Immunol. 2006, 63, 321–329. [Google Scholar] [CrossRef]
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; Ahmadian, M.R.; Moll, J.M.; Scheller, J. The balance of interleukin (IL)-6, IL-6.soluble IL-6 receptor (sIL-6R), and IL-6.sIL-6R.sgp130 complexes allows simultaneous classic and trans-signaling. J. Biol. Chem. 2018, 293, 6762–6775. [Google Scholar] [CrossRef]
- Jostock, T.; Mullberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Garbers, C.; Thaiss, W.; Jones, G.W.; Waetzig, G.H.; Lorenzen, I.; Guilhot, F.; Lissilaa, R.; Ferlin, W.G.; Grotzinger, J.; Jones, S.A.; et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011, 286, 42959–42970. [Google Scholar] [CrossRef]
- Morieri, M.L.; Passaro, A.; Zuliani, G. Interleukin-6 “Trans-Signaling” and Ischemic Vascular Disease: The Important Role of Soluble gp130. Mediat. Inflamm. 2017, 2017, 1396398. [Google Scholar] [CrossRef]
- Rabe, B.; Chalaris, A.; May, U.; Waetzig, G.H.; Seegert, D.; Williams, A.S.; Jones, S.A.; Rose-John, S.; Scheller, J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood 2008, 111, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Lesina, M.; Kurkowski, M.U.; Ludes, K.; Rose-John, S.; Treiber, M.; Kloppel, G.; Yoshimura, A.; Reindl, W.; Sipos, B.; Akira, S.; et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011, 19, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, J.; Muller, M.; Baumann, N.; Reichert, M.; Heneweer, C.; Bolik, J.; Lucke, K.; Gruber, S.; Carambia, A.; Boretius, S.; et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology 2017, 65, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J. Bioassays for growth factors. Dev. Biol. Stand. 1999, 97, 21–27. [Google Scholar] [PubMed]
- Meager, A. Measurement of cytokines by bioassays: Theory and application. Methods 2006, 38, 237–252. [Google Scholar] [CrossRef]
- Thorpe, R.; Wadhwa, M.; Page, C.; Mire-Sluis, A. Bioassays for the characterisation and control of therapeutic cytokines; determination of potency. Dev. Biol. Stand. 1999, 97, 61–71. [Google Scholar]
- Finbloom, D.S. The role of the bioassay in the evaluation of cytokines and growth factors for regulatory decision making. Dev. Biol. Stand. 1999, 97, 89–93. [Google Scholar]
- Tenhumberg, S.; Waetzig, G.H.; Chalaris, A.; Rabe, B.; Seegert, D.; Scheller, J.; Rose-John, S.; Grotzinger, J. Structure-guided optimization of the interleukin-6 trans-signaling antagonist sgp130. J. Biol. Chem. 2008, 283, 27200–27207. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Falker, K.; Kumawat, A.K.; Paramel, G.; Grenegard, M.; Sirsjo, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef]
- Retnoningrum, D.S.; Santika, I.W.M.; Kesuma, S.; Ekowati, S.A.; Riani, C. Construction and Characterization of a Medium Copy Number Expression Vector Carrying Auto-Inducible dps Promoter to Overproduce a Bacterial Superoxide Dismutase in Escherichia coli. Mol. Biotechnol. 2019, 61, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Friehs, K. Plasmid copy number and plasmid stability. Adv. Biochem. Eng. Biotechnol. 2004, 86, 47–82. [Google Scholar] [PubMed]
- Jones, K.L.; Kim, S.W.; Keasling, J.D. Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab. Eng. 2000, 2, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hawe, A.; Wiggenhorn, M.; van de Weert, M.; Garbe, J.H.; Mahler, H.C.; Jiskoot, W. Forced degradation of therapeutic proteins. J. Pharm. Sci. 2012, 101, 895–913. [Google Scholar] [CrossRef]
- Lin, J.; Su, G.; Su, W.; Zhou, C. Progress in digital PCR technology and application. Sheng Wu Gong Cheng Xue Bao 2017, 33, 170–177. [Google Scholar]
- Basu, A.S. Digital Assays Part I: Partitioning Statistics and Digital PCR. Slas. Technol. 2017, 22, 369–386. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, P.; Oppmann, B.; Voltz, N.; Fischer, M.; Rose-John, S. A role for the immunoglobulin-like domain of the human IL-6 receptor. Intracellular protein transport and shedding. Eur. J. Biochem. 1999, 263, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Muromoto, R.; Kuroda, M.; Togi, S.; Sekine, Y.; Nanbo, A.; Shimoda, K.; Oritani, K.; Matsuda, T. Functional involvement of Daxx in gp130-mediated cell growth and survival in BaF3 cells. Eur. J. Immunol. 2010, 40, 3570–3580. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |




| Experiment Parameters | Optimized Values |
|---|---|
| Pre-incubation time of sgp130-Fc with IL-6/sIL-6Rα | 1 h |
| IL-6 and sIL-6Rα working concentration | 0.5 μg/mL IL-6 and 0.25 μg/mL sIL-6Rα |
| Cell number (per well) | 30,000 |
| Action time of sgp130-Fc | 7 h |
| FBS working concentration | 10% |
| Detection range of sgp130-Fc | 40–5000 ng/mL |
| IC50, ng/mL | Day 1 | Day 2 | Day 3 | Mean | Inter-Assay CV (%) |
|---|---|---|---|---|---|
| Plate 1 | 487 | 463 | 505 | 485 | 4.3 |
| Plate 2 | 501 | 495 | 491 | 496 | 1.0 |
| Plate 3 | 494 | 484 | 486 | 488 | 1.1 |
| Mean | 494 | 481 | 494 | / | / |
| Intra-assay CV (%) | 1.2 | 3.4 | 2.0 | / | / |
| Test No. | Relative Potency of Test Sample (%) | Relative Potency of Recovery Sample (%) | Recovery Rate |
|---|---|---|---|
| 1 | 116.7 | 111.3 | 105.9% |
| 2 | 106.2 | 106.2 | 106.2% |
| 3 | 109.9 | 102 | 94.1% |
| 4 | 98.3 | 99.4 | 100.6% |
| 5 | 99.1 | 102 | 104.9% |
| 6 | 100.9 | 102.9 | 104.9% |
| Mean | 105.2 | 104.0 | 102.8% |
| Inter-assay CV | 6.8% | 4.0% | 4.6% |
| Passage of Cells | Luciferase (Copies/ug Genome) | GAPDH (Copies/ug Genome) | Luciferase Copies per Copy GAPDH | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | Mean | 1 | 2 | Mean | ||
| P10 | 44.99 | 44.09 | 44.54 | 481.92 | 473.67 | 477.79 | 0.093 |
| P30 | 52.83 | 52.98 | 52.91 | 557.69 | 549.51 | 553.60 | 0.096 |
| P60 | 45.95 | 46.99 | 46.47 | 488.39 | 479.62 | 484.00 | 0.095 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Jia, C.; Yao, W.; Pei, D.; Qin, X.; Rao, C.; Wang, J. Development and Validation of a Reporter-Cell-Line-Based Bioassay for Therapeutic Soluble gp130-Fc. Molecules 2019, 24, 3845. https://doi.org/10.3390/molecules24213845
Yu L, Jia C, Yao W, Pei D, Qin X, Rao C, Wang J. Development and Validation of a Reporter-Cell-Line-Based Bioassay for Therapeutic Soluble gp130-Fc. Molecules. 2019; 24(21):3845. https://doi.org/10.3390/molecules24213845
Chicago/Turabian StyleYu, Lei, Chuncui Jia, Wenrong Yao, Dening Pei, Xi Qin, Chunming Rao, and Junzhi Wang. 2019. "Development and Validation of a Reporter-Cell-Line-Based Bioassay for Therapeutic Soluble gp130-Fc" Molecules 24, no. 21: 3845. https://doi.org/10.3390/molecules24213845